DEFINITION
What is mesothelioma?
Mesothelioma is a form of cancer that occurs at the thin lining that covers the internal layer of several organs and body cavities, this layer is called the mesothelium. Thus mesothelioma is a conjunction of two words, mesothelium, and oma, which is usually used to describe the end process of dysplasia (the abnormal development or growth of a tissue or organ). Mesothelioma is a rare and aggressive malignancy that most often happens to the lining of the lungs and chest wall (pleura), less commonly to the lining of abdomen (peritoneum) and rarely at the sac of the heart (pericardium) and testicles. Most common symptoms of malignant pleural mesothelioma are shortness of breath (dyspnea), due to fluid gathering around the lungs (pleural effusion) and non pleuritic chest wall pain. Other symptoms may include fever, sweats, weight loss, getting easily fatigued, unexplainable weight loss, chest discomfort, and pleuritic pain. Asymptomatic patients is usually identified upon percussion and auscultation during a physical examination performed by a physician or findings obtained through a chest radiograph.
Subtopics in this article :
Mesothelioma is more commonly diagnosed in men than women and rarely affects people younger than 45. This is because mesothelioma often takes decades to develop, and men are more likely to work in jobs where asbestos exposure occurs. More than 80% of mesothelioma cases are caused by exposure to asbestos. Other causes may include genetic disposition, irradiation of the chest and abdomen, exposure to erionite (a fibrous silicate zeolite having similar traits as asbestos), and the usage of intrapleural thorium dioxide (thorotrast) as a contrast medium.
Subtopics in this article :
Mesothelioma is more commonly diagnosed in men than women and rarely affects people younger than 45. This is because mesothelioma often takes decades to develop, and men are more likely to work in jobs where asbestos exposure occurs. More than 80% of mesothelioma cases are caused by exposure to asbestos. Other causes may include genetic disposition, irradiation of the chest and abdomen, exposure to erionite (a fibrous silicate zeolite having similar traits as asbestos), and the usage of intrapleural thorium dioxide (thorotrast) as a contrast medium.
What is asbestosis?
Asbestosis is a scarring (fibrosis) and chronic inflammatory disease which affects the tissue of the lungs that is caused by exposure, inhalation and retention of asbestos fibers. Asbestosis symptoms can range from mild to severe, and usually don't appear until many years after continued exposure. The word asbestos comes from the Greek language, meaning “inextinguishable”. This condition may also be known as pneumoconiosis, or to be more precise, pneumoconiosis caused by asbestos inhalation. Asbestos refers to a group of naturally occurring hydrated mineral silicate fibers including two major forms: serpentine, represented by chrysotile (white asbestos) and amphibole, which includes crocidolite (blue asbestos), amosite (brown asbestos), anthophyllite, actinolite and tremolite. Asbestos is a whitish material that was used in buildings for insulation, flooring and roofing in the past, but is now no longer used. While asbestos can be dangerous, it doesn't present a health risk if left undisturbed. But if material containing asbestos is damaged, it can release a fine dust that contains asbestos fibres. People with extensive occupational exposure to the mining, manufacturing, handling, or removal of asbestos are at risk of developing asbestosis, therefor it is considered an occupational lung disease. When the dust is breathed in, the asbestos fibres enter the lungs and can gradually damage them over time. But you would need prolonged exposure to asbestos fibres, usually over many years, before you develop asbestosis.
The signs and symptoms of asbestosis typically manifest after a significant amount of time has passed following asbestos exposure, often several decades. The primary symptom of asbestosis is generally the slow onset of shortness of breath, especially with physical activity. Clinically advanced cases of asbestosis may lead to respiratory failure. Other symptoms may include coughing, chest pain, blood in the sputum, difficulty in swallowing, loss of appetite and weight loss. When a physician listens with a stethoscope to the lungs of a person with asbestosis, they may hear inspiratory crackles. A considerable note is that asbestosis specifically refers to fibrosis within the lung tissue from asbestos, and not scarring around the outside of the lungs.
So in short, mesothelioma is an end result cancer that is mostly caused by asbestosis (exposure to asbestos)
What is the difference between asbestos cancer and mesothelioma?
Even though the term “asbestos cancer” most often refers to mesothelioma, because the majority of severe asbestosis results in mesothelioma, a number of other cancers are associated with asbestos exposure. Lung cancer can be directly caused by asbestos exposure, and some studies have suggested a link between exposure and other types of cancer. Elevated risks for a number of other cancers continue to be investigated. According to the World Health Organization, approximately half of all deaths from occupational cancer are caused by asbestos.
In a large study of 1,047 asbestos industry employees, a malignant tumor was listed as the official cause of death for 208 workers. Respiratory cancers (primarily in the bronchus, trachea or lung) made up the majority of the cancer deaths, followed by cancers of the digestive organs and peritoneum, the lining of the abdomen.
Aside from mesothelioma and lung cancer, asbestos has been associated with a number of other cancers. Research is still determining the extent to which asbestos can cause other types. Other cancers that are potentially associated with asbestos exposure, although in various rates and significance are leukemia, gastrointestinal, kidney, colorectal, breast, prostate, and gallbladder cancers, and Hodgkin's and Non-Hodgkin’s Lymphoma. Further explanation concerning this matter will be later discussed below at the suitable sub-topic.
ETIOLOGIES (Causes)
A. Asbestos exposure
Asbestos, particularly the types of amphibole asbestos known as crocidolite and amosite asbestos, is the main carcinogen implicated in the pathogenesis of malignant pleural mesothelioma. Exposure to chrysotile asbestos is also known to be associated with malignant mesothelioma, but at a lower incidence than occurs with the other types. (The rod-shaped amphiboles are more carcinogenic than the chrysotile. Further discussed below in types and categories)
Approximately 8 million people in the United States have been exposed to asbestos in the workplace. A substantial proportion of patients with malignant pleural mesothelioma were exposed to asbestos in asbestos mills, mines, shipping yards, or their homes. The crocidolite in asbestos is associated with mesothelioma in miners, manufacturers who use asbestos, and heating and construction workers. Family members of workers exposed to asbestos can also be at risk of exposure if asbestos becomes embedded in the workers’ clothing. The greater the exposure the greater the risk. As of 2013 about 125 million people have been exposed to asbestos at work worldwide. High rates of disease occur in people who mine asbestos, produce products from asbestos, work with asbestos products, live with asbestos workers, or work in buildings containing asbestos. Asbestos exposure and the onset of cancer are generally separated by about 40 years. Washing the clothing of someone who worked with asbestos could also increases the risk. In 2015 about 60,800 people had mesothelioma and 32,000 died from the disease. Rates of mesothelioma vary in different areas of the world. Rates are higher in Australia, the United Kingdom, and lower in Japan. It occurs in about 3,000 people per year in the United States. It occurs more often in males than females. Rates of disease have increased since the 1950s. Diagnosis typically occurs after the age of 65 and most deaths occur around 70 years old. The disease was rare before the commercial use of asbestos.
Some of the industries associated with asbestos exposure include :
- Mining
- Ceramics
- Paper milling
- Auto parts (asbestos brake lining)
- Ship building involving the use of asbestos
- Asbestos cement manufacture
- Railroad repair
- Insulation
In Turkey, the use of the fibrous substance erionite (similar to amphibole asbestos) in building construction has led to an epidemic of pulmonary mesothelioma. Environmental exposure to asbestos in areas polluted by the substance may also increase the incidence of mesothelioma.
Alcohol, dietary factors, and tobacco smoke have not been found to have any effect on the incidence of pleural mesothelioma.
Types of asbestos fibers
The word asbestos derives from the Greek language and means inextinguishable. The term refers to a group of naturally occurring, heat-resistant fibrous silicates, the fibers of which are long and thin (length-to-diameter ratio >3) and either curved or straight. The curved fibers make up serpentine asbestos (chrysotile is the prime example), and the straight fibers make up amphibole asbestos.
Six mineral types are defined by the United States Environmental Protection Agency (EPA) as "asbestos" including those belonging to the serpentine class and those belonging to the amphibole class. All six asbestos mineral types are known to be human carcinogens. The visible fibers are themselves each composed of millions of microscopic "fibrils" that can be released by abrasion and other processes. Mainly, asbestos fibers are divided into two classes or categories, the serpentine and amphibole class/category.
1. Serpentine Class Asbestos
Serpentine class fibers are curly. Chrysotile is the only member of the serpentine class. Chrysotile is the only type of asbestos that is from the serpentine family and is known as white asbestos. These fibers are curly and are comprised of sheets of crystals. Throughout industrial history, more than 95 percent of all asbestos used around the world was of the chrysotile variety. In many countries where other types of asbestos have been banned, the “controlled use” of chrysotile is still permitted. Despite the numerous studies that have proven chrysotile carcinogenic traits, this exception is the result of a long lobbying history by those in the asbestos industry.
Chrysotile
Chrysotile, is obtained from serpentinite rocks which are common throughout the world. Its idealized chemical formula is Mg3(Si2O5)(OH)4. Chrysotile appears under the microscope as a white fiber.
Chrysotile has been used more than any other type and accounts for about 95 percent of the asbestos found in buildings in America. Chrysotile is one of the most common and most dangerous forms of asbestos found within our Earth. This type of asbestos accounts for approximately 90 percent of commercially-used asbestos in the world. Chrysotile asbestos fibers are long, white, and curly. Chrysotile is more flexible than amphibole types of asbestos, and can be spun and woven into fabric. The most common use was corrugated asbestos cement roofing primarily for outbuildings, warehouses and garages. It may also be found in sheets or panels used for ceilings and sometimes for walls and floors. Chrysotile has been a component in joint compound and some plasters. Numerous other items have been made containing chrysotile including brake linings, fire barriers in fuseboxes, pipe insulation, floor tiles, residential shingles, and gaskets for high temperature equipment.
Many studies have proven that exposure to chrysotile asbestos, commonly referred to as white asbestos, can cause a number of serious health conditions. While most commercial uses of asbestos in the United States have been of the chrysotile type, the use of this toxic mineral has declined significantly during the last few decades.
Naturally occurring deposits of chrysotile are often accompanied by trace amounts of tremolite (amphibole) asbestos, which is considered more toxic than chrysotile. However, several reports have indicated that exposure to solely chrysotile asbestos fibers can occur and such exposure can be equally hazardous as exposure to amphibole asbestos types.
Scientists from the National Institute for Occupational Safety and Health concluded that chrysotile asbestos should be treated with virtually the same level of concern as the amphibole forms of asbestos.
Uses of Chrysotile Asbestos
In comparison to amphiboles, chrysotile fibers are generally finer with high flexibility and good heat resistance. Known as the most common asbestos mineral, chrysotile accounts for about 90 to 95 percent of asbestos used in commercial applications in the United States.
This toxic mineral has been utilized in a number of products, including:
- Cement
- Gaskets
- Insulation
- Joint compound
- Roofing materials
- Brake pads
- Brake linings
2. Amphibole Class Asbestos
Amphibole class fibers are needle-like. The other five types of asbestos are classified in the amphibole class. Amosite (brown asbestos) and crocidolite (blue asbestos) are considered the most commercially valuable types. Anthophyllite, tremolite and actinolite are the other non-commercial forms of amphibole asbestos. All amphibole fibers are straight and longer than chrysotile fibers, and studies suggest it may take less exposure to amphibole asbestos to cause mesothelioma than chrysotile asbestos.
Amphiboles including amosite (brown asbestos) and crocidolite (blue asbestos) were formerly used in many products until the early 1980s. Tremolite asbestos constituted a contaminant of many if not all naturally occurring chrysotile deposits. The use of all types of asbestos in the amphibole group was banned in much of the Western world by the mid-1980s, and in Japan by 1995. Some products that included amphibole types of asbestos included the following:
- Low density insulating board (often referred to as AIB or asbestos insulating board) and ceiling tiles;
- Thermal and chemical insulation (e.g., fire rated doors, limpet spray, lagging and gaskets).
- Asbestos-cement pipe (made until the early 1990s by at least one manufacturer);
- Asbestos-cement sheets and pipes for construction, casing for water and electrical/telecommunication services;
Some cigarette manufacturers used crocidolite asbestos in its "Micronite" filter from 1952 to 1956. While mostly chrysotile asbestos fibers were once used in automobile brake pads, shoes, and clutch discs, contaminants of amphiboles were present. Since approximately the mid-1990s, brake pads, new or replacement, have been manufactured instead with linings made of ceramic, carbon, metallic and aramid fiber (Twaron or Kevlar—the same material used in bulletproof vests).
Artificial Christmas snow, known as flocking, was previously made with asbestos. It was used as an effect in films including The Wizard of Oz and department store window displays and it was marketed for use in private homes under brand names that included "Pure White", "Snow Drift" and "White Magic".
a. Crocidolite asbestos
Crocidolite or blue asbestos, is the fibrous form of the amphibole riebeckite, found primarily in southern Africa, but also in Australia and Bolivia. One formula given for crocidolite is Na2Fe2+3Fe3+2Si8O22(OH)2. Crocidolite is seen under a microscope as a blue fiber.
Crocidolite commonly occurs as soft friable fibers. Asbestiform amphibole may also occur as soft friable fibers but some varieties such as amosite are commonly straighter. All forms of asbestos are fibrillar in that they are composed of fibers with breadths less than 1 micrometer in bundles of very great widths. Asbestos with particularly fine fibers is also referred to as "amianthus". Crocidolite takes the form of blue, straight fibers. It is a sodium iron magnesium silicate, and is considered to be the most dangerous type of asbestos due to its physical properties.
Multiple asbestos studies suggest crocidolite may be responsible for more deaths than any other type of asbestos because its fibers are so thin — about the diameter of a strand of hair. When airborne, these fibers can be inhaled easily and become lodged in the lining of the lungs, more so than other forms of asbestos forms. Once inside the body, the fibers do not break down easily. This can lead to potentially life-threatening lung and abdominal conditions, including lung cancer, mesothelioma and asbestosis.
The existence of crocidolite asbestos was first established in the early 1800s in South Africa. At the time, the mineral was known as “wooly stone,” but interest in the naturally occurring mineral didn’t take off until the 1880s, and large mining efforts of the material didn’t begin until the early 1900s.
An estimated 18 percent of crocidolite miners die from mesothelioma, research shows, and people living near crocidolite mines may also have increased risks for mesothelioma and other diseases.
Crocidolite is also known as “blue” asbestos. This form can be translucent or nearly opaque (which means light can’t penetrate it). The most common mining sites for this type of asbestos were Bolivia, Australia and southern Africa.
Today, crocidolite mining has virtually ceased because of both physical limitations and serious health risks. Crocidolite-containing materials are also more brittle than other amphibole asbestos products, meaning they break down sooner and can more readily lead to asbestos exposure.
Crocidolite is categorized as an amphibole, which is usually a needle-like mineral that forms in crystal groupings, either as fibers or columns. Typically, crocidolite fibers can be curved or straight. While brittle, the fibers are flexible enough to bend beyond 90 degrees before breaking.
Uses of Crocidolite Asbestos
Like other types of asbestos, crocidolite was used to make a number of commercial and industrial products. It did have a drawback that other asbestos types did not: It is less heat-resistant, making it less useful for industrial manufacturing.
Some of the leading uses of crocidolite asbestos included:
- Ceiling tiles
- Cement sheets containing asbestos
- Electrical or telecommunication wires
- Fire protection
- Insulation boards
- Water encasement (enclosing)
- Chemical insulation
- Spray-on insulation
- Acid storage battery casings
- Thermal insulation (lagging and gaskets)
- Millboards (commercial ovens and steam pipes)
b. Amosite asbestos
Amosite, often referred to as brown asbestos, is a trade name for the amphiboles belonging to the cummingtonite-grunerite solid solution series, commonly from South Africa, named as a partial acronym for "Asbestos Mines of South Africa". Amosite asbestos is recognized by its straight fibers and brown color. Amosite asbestos contains iron and magnesium, and was most used within different types of insulation products. The EPA has determined amosite to be the second most used type of asbestos in the United States. One formula given for amosite is Fe7Si8O22(OH)2. Amosite is seen under a microscope as a grey-white vitreous fiber. It is found most frequently as a fire retardant in thermal insulation products, asbestos insulating board and ceiling tiles.
According to the American Cancer Society, exposure to amosite asbestos creates a higher risk of cancer in comparison with other types of asbestos. Several asbestos studies suggest exposure to amosite can cause lung cancer, mesothelioma and asbestosis.
In its natural state, amosite is known as the mineral grunerite. Commercially, grunerite is referred to as amosite or brown asbestos. Approximately 80,000 tons of amosite were mined in the Transvaal province of South Africa by 1970.
Uses of Amosite
Amosite asbestos offer good tensile strength and heat resistance. Commercial products that have been manufactured with amosite include:
- Roofing products
- Fire protection
- Gaskets, lagging
- Cement sheets
- Thermal insulation
- Plumbing insulation
- Insulation boards
- Chemical insulation
- Electrical insulation
- Tiles, including those for ceilings, roofs and floors
The U.S. Environmental Protection Agency has determined amosite to be the second most commonly used mineral type of asbestos in the United States.
c. Vermiculite asbestos
Vermiculite asbestos is a mineral that expands when heated, a process called “exfoliation” or “popping.” This process forms a light-weight material used for industrial purposes including insulation, packing materials and soil improvement. Because vermiculite can contain large amounts of tremolite, exposure may increase a person’s risk of developing an asbestos-related disease.
Vermiculite is a hydrated laminar magnesium-aluminum-iron silicate which resembles mica. It can be used for many industrial applications and has been used as insulation. Some deposits of vermiculite have been found to be contaminated with small amounts of asbestos.
One vermiculite mine in Libby, Montana exposed workers and community residents to danger by mining vermiculite contaminated with asbestos, typically richterite, winchite, actinolite or tremolite. Vermiculite contaminated with asbestos from the Libby mine was used as insulation in residential and commercial buildings through Canada and the United States. The loose-fill vermiculite in this mine was marketed as Zonolite but was also used in sprayed-on products such as Monokote.
In 1999 the EPA began cleanup efforts in Libby and now the area is a Superfund cleanup area. The EPA has determined that harmful asbestos is released from the mine as well as through other activities that disturb soil in the area.
This health risk is illustrated by the mining and milling operations in Libby, Montana, one of the United States’ largest sources of vermiculite. The tremolite-contaminated vermiculite at Libby was sold as Zolonite attic insulation, which the EPA estimates could be in millions of American homes. Because more than 70 percent of the vermiculite sold in the United States between 1919 and 1990 came from Libby, professionals recommend treating all vermiculite insulation as if it is contaminated with tremolite.
While some asbestos-containing vermiculite mines have been shut down in recent years, many vermiculite products that contain asbestos are still in use today.
Over the last century, vermiculite has been widely mined and processed worldwide for various construction, industrial and horticultural applications, as it is a superior insulator and filler material that is both lightweight and inexpensive.
Vermiculite compounds have been used for the following applications:
Vermiculite compounds have been used for the following applications:
- Fertilizer carrier
- Potting soil additive
- Soil conditioner
- Fireproofing material
- Whitewashes
- Attic insulation (loose-fill, commonly sold under the product name Zonolite)
- Acoustic finishes
- Spray-on insulation
- Concrete mixes for swimming pools
- Stucco
- Alternative to gypsum wallboard
- Plasterboard
- Packaging material (similar to styrofoam peanuts)
d. Tremolite asbestos
Tremolite is an amphibole asbestos. Tremolite fibers have been useful for commercial products because they are strong, flexible, heat-resistant, and can be spun and woven into cloth. The known formula for trmolite is Ca2Mg5Si8O22(OH)2. Tremolite asbestos fibers can be brown, gray, white or green, and like other types, can also be translucent. Tremolite was not mined or used commercially on its own, but could often be found contaminating other minerals, such as chrysotile, vermiculite and talc.
Tremolite was used in a variety of commercial and industrial products because of its ability to insulate and fireproof materials. Some of the more common products that contained tremolite included:
- Roofing materials
- Plumbing materials
- Paints
- Sealants
- Insulation
Tremolite contains calcium, magnesium, silicon, hydrogen and oxygen. The mineral can be brown, gray, white or green and may appear to be transparent.
Minerals That Contain Tremolite
Rarely mined on its own, tremolite is often found in large amounts of other minerals such as talc and vermiculite. Researchers found that talc miners and millers are at higher risk for developing lung cancer and other respiratory conditions. When these minerals are used for industrial purposes, exposure to asbestos becomes a concern.
Talc
Talc is the softest known mineral on earth and is used for myriad industrial purposes including chalk, paints, rubber, cosmetics, ceramics and pharmaceuticals (for lung function). Most famously, this mineral is used for making talcum powder. Since 1973, U.S. laws require all commercial talcum products to be asbestos-free.
Talc can sometimes be contaminated with asbestos due to the proximity of asbestos ore (usually tremolite) in underground talc deposits. By 1973, US federal law required all talc products to be asbestos-free, and today there is strict quality control in the production of talc products, separating cosmetic-grade talc (e.g. talcum powder) from industrial-grade talc (often used in friction products) has largely eliminated this issue for consumers.
In 2000, tests in a certified asbestos-testing laboratory found the tremolite form of amphibole asbestos in three out of eight bigger brands of children's crayons that are made partly from talc. Overall, 32 different types of crayons from these brands contained more than trace amounts of asbestos, and eight others contained trace amounts. Although there has been disputes between the manufacturers, the mining company which provided the talc the crayon makers, the United States Mine Safety and Health Administration (MSHA). In June 2000, these major crayon companies agreed to stop using talc in their products, and changed their product formulations in the United States.
e. Actinolite asbestos
Actinolite is an amphibole that is generally dark in color. Actinolite (pronounced ak-TIN-uh-lyte) can appear in multiple forms such as dense and compact or brittle and fibrous, along with different colors, including white, gray, brown or green. The mineral’s name stems from the Greek “aktinos,” meaning “ray” or “beam,” stemming from its radiating fibrous form. The formula for actinolite is Ca2(Mg, Fe)5(Si8O22)(OH)2. Actinolite asbestos appears as dark green crystals or fibrous aggregates. Like tremolite and anthophyllite, actinolite asbestos is often found as a contaminate within different commercial asbestos products. It has been found in paints, sealants, children's toys, and more.
- Calcium
- Magnesium
- Iron
- Silicon
- Oxygen
- Hydrogen
Uses of Actinolite Asbestos
Actinolite is typically used with the similar mineral vermiculite, which expands when heated. Vermiculite and actinolite make for an effective, light-weight insulation material. Other common uses for actinolite and vermiculite include:
- Insulation material
- Gardening
- Concrete materials used in construction
- Structural fire-proofing
f. Anthophyllite Asbestos
Anthophyllite asbestos is known to cause asbestos-related diseases, but most studies indicate the risk of developing mesothelioma from anthophyllite exposure is much less than it is from exposure to other types of asbestos. The known formula for anthophyllite is (Mg, Fe)7Si8O22(OH)2. Like tremolite asbestos, anthophyllite minerals were not sought out for their commercial use, but instead found their way into products made with vermiculite and talc. The miners of vermiculite and talc are at high risk for developing asbestos-related diseases because of anthophyllite contamination within the substances they mined. Anthophyllite asbestos can range in color from white to gray to brown.
Although it took much longer for anthophyllite to be recognized as a mesothelioma risk than it did for amosite, chrysotile and crocidolite asbestos, there is a clear connection from exposure to it and to the development of mesothelioma.
Anthophyllite is one of the rarest types of asbestos and does not have a long history of commercial use. The mining of this mineral began in Finland in 1890. Since then, smaller deposits were mined in North Carolina and Georgia.
While considered to be among the noncommercial types of asbestos, anthophyllite has been used in products containing minerals such as vermiculite and talc.
Traces of anthophyllite may be present in talc and related products such as talcum powder.
g. Other asbestiform materials
Other natural asbestiform minerals, such as richterite, Na(CaNa)(Mg, Fe++)5(Si8O22)(OH)2, and winchite, (CaNa)Mg4(Al, Fe3+)(Si8O22)(OH)2, though not regulated, are said by some to be no less harmful than tremolite, amosite, or crocidolite. They are termed "asbestiform" rather than asbestos. Although the U.S. Occupational Safety and Health Administration (OSHA) has not included them in the asbestos standard, NIOSH and the American Thoracic Society have recommended them for inclusion as regulated materials because they may also be hazardous to health.
B. Genetic Disposition
Because only a small number of people exposed to asbestos develop mesothelioma, scientists believe genetics can play a role in a person's risk. Researchers have confirmed a mutation in a gene called BAP1 increases the likelihood of developing mesothelioma and other cancers. If someone else in your family has mesothelioma, genetics suggest you have an increased risk for developing the cancer. In a recent research carried on white American population in 2012, it was found that people with a germline mutation in their BAP1 gene are at higher risk of developing mesothelioma and uveal melanoma.
Most malignant mesotheliomas have complex karyotypes, with extensive aneuploidy and the rearrangement of many chromosomes. Loss of 1 copy of chromosome 22 is the single most common karyotypic change in malignant mesothelioma. Other chromosomal changes commonly observed include deletions in the chromosome arms 1p, 3p, 9p, and 6q. Several changes in the tumor suppressor genes p16 (CDKN2A) and p14 (ARF) and loss of function of neurofibromin-2 (NF2) have also been noted.
Detailed epidemiological investigation has shown that erionite causes mesothelioma mostly in families with a genetic predisposition. Erionite is a zeolite mineral with similar properties to asbestos and is known to cause mesothelioma. Erionite is found in deposits in the Western United States, where it is used in gravel for road surfacing, and in Turkey, where it is used to construct homes. In Turkey, the United States, and Mexico, erionite has been associated with mesothelioma and has thus been designated a "known human carcinogen" by the US National Toxicology Program.
C. Other Etiologies of Mesothelioma
Interleukin-8 has direct growth-potentiating activity in mesothelial cell lines. Malignant mesothelioma has also been linked to therapeutic radiation using thorium dioxide and zeolite, a silicate in the soil. In rare cases, mesothelioma has also been associated with irradiation of the chest or abdomen, intrapleural thorium dioxide (thorotrast) as a contrast medium, and inhalation of other fibrous silicates, such as erionite or talc.
Several case reports have documented mesothelioma in patients who received radiation to the thorax or abdomen. Cancer patients who are treated with radiotherapy have shown increased risks for mesothelioma and the average interval between radiotherapy and mesothelioma was 21 years. Animal studies using rats also support the role of radiation as a causative factor of mesothelioma.
There is no evidence showing an association between mesothelioma and smoking. There are some anecdotal case reports suggesting that chronic inflammation and scaring of the pleura, intrapleural thorium dioxide, and some chemicals may cause mesothelioma.
Some studies suggest people who received a polio vaccine between 1955 and 1963 may have an increased risk of developing mesothelioma. Tens of millions of polio vaccines during that nine-year span were infected by the simian virus 40 (SV40). Although the largest studies did not find a link between the virus and increased mesothelioma risk, the topic remains controversial as studies continue.
Some studies suggest that simian virus 40 (SV40) may act as a cofactor in the development of mesothelioma. This has been confirmed in animal studies, but studies in humans are inconclusive. An etiologic role for simian virus 40 in malignant mesothelioma has been suggested. However, although asbestos exposure alone has been associated with malignant mesothelioma, simian virus 40 alone has not. Thus, some epidemiologic evidence exists that simian virus 40 is a possible cocarcinogen. Its direct role at this point is still controversial.
PATHOPHYSIOLOGY
The mesothelium consists of a single layer of avascular flat nucleated cells that lines serosal cavities and the majority of internal organs, playing important roles in maintaining normal serosal integrity and function. The exact mechanism of mesothelioma development – a highly aggressive tumor with a dismal prognosis – is still not comprehensibly understood.
The pathogenesis of mesothelioma in humans is predicated on the transport of asbestos fibers to the pleura of the lungs – the fluid-filled sac that surrounds the lungs. These fibers induce an immune system response, by macrophages and other specialized cells, to the lesions that occur as a result of asbestos-fiber irritation of tissues. These lesions continue to attract, and aggregate, specialized cells, causing cellular changes within the lesion that terminate in a malignant tumor.
Evidence gathered from animal experiments indicates that asbestos acts as a complete carcinogen, though the molecular mechanisms of malignancy are not entirely clear. However, research indicates that asbestos fibers act directly on chromosomes, or structural proteins within the cell wall, to effect complex changes, with chromosome 22 showing the most distinct changes. These changes can lead to either deletion of tumor suppressing genes or the prevention of apoptosis (programmed cell death) or activation of oncogenes, via the interposition of foreign DNA, which asbestos appears to facilitate.
Some of the malignancy pathways that have been suggested are:
- Oxygen-free radicals like hydroxyls released by macrophages which interact with chromosomal material.
- Growth factors triggering mesothelial cell proliferation
- Immunosuppressive factors integral to asbestos which may reduce the production of lymphocytes
Four-fifths of the malignant mesotheliomas in humans result from higher than normal exposure to asbestos either through occupational or regional exposure, though recent studies suggest a genetic factor that predisposes some individuals in Turkey to mesothelioma. In addition, natural serpentine rock formations around the world predicate higher-than-normal occurrences of mesothelioma.
Of the estimated two billion mesothelial cells which form the linings of body cavities like the pleura, peritoneum, pericardium, and tunica (which protects the internal reproductive organs in both sexes), all are affected by four principal processes which can lead to malignancy. These are: irritation, mitotic disruption, DNA damage via iron-rich oxygen radicals, and the phosphorylation of the mitogen-activated protein (MAP) kinases which are involved in cell differentiation and apoptosis.
The pathophysiological or pathogenic mechanism has been linked to asbestos fibers such as curly, serpentine fibers (white asbestos) or long chain-like fibers such as amosite (brown asbestos), crocidolite (blue asbestos), anthophyllite, tremolite and actinolite. The pleura represents the target for the carcinogenic activity of asbestos due to the fact that asbestos can efficiently move from the lung to the pleural space, concentrating in the parietal pleura at the sites of lymphatic drainage.
Of the two basic fiber types of asbestos, the larger are the most carcinogenic, due to their greater persistence in the body and their higher iron content, which promote higher production of reactive oxygen radicals. However, less than 10 percent of individuals exposed to asbestos at higher doses over long periods of time actually develop malignant pleural mesothelioma, or MPM, leading researchers to search for other etiologies, specifically a genetic component, a viral component (SV40) implicated in various other cancers (and formerly used in the Salk polio vaccine), and a potential link with hyaluronic acid, which is a definitive diagnostic cell stain for differentiating mesothelioma from adenocarcinomas.
The association between amphibole asbestos exposure and mesothelioma development is well accepted. In particular, crocidolite is generally considered to be the most oncogenic type of asbestos. The long and thin fibers (especially ≥ 8 µm in length ≤ 0.25 µm in width) are thought to be more dangerous, because they have longer biopersistance in the pleura. These fibers are able to penetrate the lung and cause repeated damage, tissue repair and local inflammation.
Chrysotile is the most common type of asbestos and accounts for about 90% of the world’s asbestos production. Whether chrysotile causes mesothelioma is still controversial. Some scientists suggested that chrysotile plays an important role in the pathogenesis of mesothelioma, because chrysotile fibers induce DNA damage and chromosome abnormalities in human and rat mesothelial cells in vitro, and cause mesothelioma in animals. Some authors suggested that chrysotile may cause mesothelioma but at a lower rate compared to amphibole asbestos. Hodgson and Darnton suggested that the exposure specific risk of mesothelioma is broadly in the ratio of 1:100:500 for chrysotile, amosite and crocidolite, respectively. Instead, Suzuki et al. proposed that chrysotile is the main contributor to the causation of mesothelioma based on their analysis of lung and mesothelial tissues taken from 168 cases of mesothelioma, in which they found that chrysotile was the most common asbestos type. Other authors have instead proposed that chrysotile does not cause mesothelioma and that it is the amphibole that often contaminates chrysotile that causes mesothelioma. Some studies show that chrysotile asbestos is considerably less biopersistent than amphibole asbestos once inhaled in the lungs, and chrysotile fibers do not cause a pronounced inflammatory response compared to the amphibole tremolite. Some of us conducted an extensive review of the literature and found that the data were so radically contradictory and, at times, flawed by conflict of interest that it was not possible to conclude whether chrysotile does or does not cause mesothelioma.
Among different types of mineral fibers, erionite is the most potent induces of mesothelioma. Erionite has been detected in the lungs of villagers in several towns in Cappadocia, Turkey, where 50% or more of deaths are caused by mesothelioma. Animal experiments showed that erionite is the most potent fiber in causing mesothelioma. Pleural mesothelioma was observed in 40 of 40 rats injected with erionite compared to 19 of 40 rats injected with asbestos. Inhalation of the erionite fibers induced a similar effect: 27/28 rats developed mesothelioma compared to only 4/124 rats exposed to crocidolite. Erionite is a zeolite mineral with similar properties to asbestos and is known to cause mesothelioma. Detailed epidemiological investigation has shown that erionite causes mesothelioma mostly in families with a genetic predisposition. Erionite is found in deposits in the Western United States, where it is used in gravel for road surfacing, and in Turkey, where it is used to construct homes. In Turkey, the United States, and Mexico, erionite has been associated with mesothelioma and has thus been designated a "known human carcinogen" by the US National Toxicology Program.
Asbestosis is the scarring of lung tissue (beginning around terminal bronchioles and alveolar ducts and extending into the alveolar walls) resulting from the inhalation of asbestos fibers.. All forms of asbestos fibers are responsible for human disease as they are able to penetrate deeply into the lungs. When such fibers reach the alveoli (air sacs) in the lung, where oxygen is transferred into the blood, the foreign bodies (asbestos fibers) cause the activation of the lungs' local immune system and provoke an inflammatory reaction dominated by lung macrophages that respond to chemotactic factors activated by the fibers. This inflammatory reaction can be described as chronic rather than acute, with a slow ongoing progression of the immune system attempting to eliminate the foreign fibers. Macrophages phagocytose (ingest) the fibers and stimulate fibroblasts to deposit connective tissue. Due to the asbestos fibers' natural resistance to digestion, some macrophages are killed and others release inflammatory chemical signals, attracting further lung macrophages and fibrolastic cells that synthesize fibrous scar tissue, which eventually becomes diffuse and can progress in heavily exposed individuals. This tissue can be seen microscopically soon after exposure in animal models. Some asbestos fibers become layered by an iron-containing proteinaceous material (ferruginous body) in cases of heavy exposure where about 10% of the fibers become coated. Most inhaled asbestos fibers remain uncoated. About 20% of the inhaled fibers are transported by cytoskeletal components of the alveolar epithelium to the interstitial compartment of the lung where they interact with macrophages and mesenchymal cells. The cytokines, transforming growth factor beta and tumor necrosis factor alpha, appear to play major roles in the development of scarring inasmuch as the process can be blocked in animal models by preventing the expression of the growth factors. The result is fibrosis in the interstitial space, thus asbestosis. This fibrotic scarring causes alveolar walls to thicken, which reduces elasticity and gas diffusion, reducing oxygen transfer to the blood as well as the removal of carbon dioxide. This can result in shortness of breath, a common symptom exhibited by individuals with asbestosis.
The predictive pathway for mesothelial pathogenesis remains a subject of intense scrutiny and considerable dispute among scientists, with only one definitive cause recognized; genetics may play as large role in the development of mesothelioma as it does in other cancers, where a gene known as p53 has been tentatively identified as the flashpoint for mutations leading to cancer.
Mesothelioma is a disease in which cells of the mesothelium become abnormal and divide without control or order. They can invade and damage nearby tissues and organs. Cancer cells can also metastasize (spread) from their original site to other parts of the body. Most cases of mesothelioma begin in the pleura or peritoneum. Mesothelioma is an aggressive malignancy caused by multiple factors that may work alone or in combination. It is hoped that the recent advances in understanding the mechanisms of mesothelioma pathogenesis will eventually lead to novel preventive and therapeutic strategies for mesothelioma patients. Importantly, some drugs that specifically target the molecular pathways that lead to mesothelioma are already available and can be tested in high-risk cohorts.
Mechanisms of asbestos pathogenesis
During the long latency period of malignant mesothelioma, a myriad of pathogenic events may occur that can contribute to the development of the disease. After asbestos fibers are inhaled deeply into the lung and penetrate pleural space, prolonged cycles of tissue damage, repair and local inflammation are initiated, following the interaction of asbestos fibers with mesothelial cells. That in turn leads to carcinogenesis.
Reactive oxygen species induced by asbestos fibers with their exposed surface lead to DNA damage and stimulate a signal transduction cascade. Macrophages phagocytize asbestos fibers, but are unable to digest them, producing in turn abundant reactive oxygen species. These events activate MAP-kinase signaling pathways through the epithelial growth factor (EGF) receptor, and several of the induced transcription factors are highly expressed in mesothelioma.
Asbestos fibers can absorb different proteins and chemicals to the broad surface of asbestos, with the accumulation of hazardous molecules (including carcinogens) as a consequence. Once inside, asbestos fibers bind important cellular proteins, thus their subsequent deficiency can also be detrimental for normal mesothelial cells.
Macrophages and asbestos-exposed mesothelial cells produce a panoply of different growth factors and cytokines which induce inflammation and promote tumor development. Those include tumor necrosis factor-α, insulin-derived growth factor-1, interleukin-1β, transforming growth factor-β, granulocyte/macrophage colony-stimulating factors and platelet-derived growth factor.
Tumor necrosis factor-α has been shown to activate nuclear factor-κB, which seriously contribute to tumor formation and progression in mesothelioma. High-mobility group box 1 protein has also been shown to be released from mesothelial cells, promoting an inflammatory response by establishing an autocrine circuit in mesothelial cells that influences their proliferation and survival.
Chromosomal aberrations
Long latency period (up to 40 years) suggest that multiple genetic alterations are important in the conversion from normal to malignant mesothelial cell. Comprehensive karyotypic analysis has revealed that malignant mesotheliomas display multiple clonal chromosomal abnormalities (more than 10 of them in most mesotheliomas).
Asbestos fibers are also engulfed by mesothelial cells, which can then disrupt mitotic spindles and influence the cell cycle process. Tangling of asbestos fibers with mitotic spindles may result in chromosomal structural abnormalities and aneuploidy of mesothelial cells.
Loss of one copy of chromosome 22 represents the single most consistent chromosomal change in patients with mesothelioma. Specific deletions of chromosomal sites involve the short arm (p) of chromosomes 1, 3 and 9, as well as the long arm (q) of chromosome 6. Other nonrandom cytogenetic alterations can be found on other chromosomes as well.
Certain tumor suppressor genes located in aforementioned chromosomal regions have also been implicated in the disease, including CDKN2A/ARF at chromosome band 9p21 and NF2 at 22q12. Mutations of the p53 gene (one of the most frequent genetic changes seen in the cancer cells) are occasionally observed in malignant mesothelioma as well.
It has also been postulated that simian virus 40 (SV40) can bind and inactivate wild-type p53 in mesothelioma, interfering with DNA repair, as well as apoptotic and growth inhibitory functions. Although it is a DNA monkey virus, the probable route of transmission to humans was through the SV40 contaminated polio vaccines distributed between 1955 and 1978.
Systemic
The mesothelium consists of a single layer of flattened to cuboidal cells forming the epithelial lining of the serous cavities of the body including the peritoneal, pericardial and pleural cavities. Deposition of asbestos fibers in the parenchyma of the lung may result in the penetration of the visceral pleura from where the fiber can then be carried to the pleural surface, thus leading to the development of malignant mesothelial plaques. The processes leading to the development of peritoneal mesothelioma remain unresolved, although it has been proposed that asbestos fibers from the lung are transported to the abdomen and associated organs via the lymphatic system. Additionally, asbestos fibers may be deposited in the gut after ingestion of sputum contaminated with asbestos fibers.
Pleural contamination with asbestos or other mineral fibers has been shown to cause cancer. Long thin asbestos fibers (blue asbestos, amphibole fibers) are more potent carcinogens than "feathery fibers" (chrysotile or white asbestos fibers). However, there is now evidence that smaller particles may be more dangerous than the larger fibers. They remain suspended in the air where they can be inhaled, and may penetrate more easily and deeper into the lungs. "We probably will find out a lot more about the health aspects of asbestos from [the World Trade Center attack], unfortunately," said Dr. Alan Fein, chief of pulmonary and critical-care medicine at North Shore-
Long Island Jewish Health System.
Mesothelioma development in rats has been demonstrated following intra-pleural inoculation of phosphorylated chrysotile fibers. It has been suggested that in humans, transport of fibers to the pleura is critical to the pathogenesis of mesothelioma. This is supported by the observed recruitment of significant numbers of macrophages and other cells of the immune system to localized lesions of accumulated asbestos fibers in the pleural and peritoneal cavities of rats. These lesions continued to attract and accumulate macrophages as the disease progressed, and cellular changes within the lesion culminated in a morphologically malignant tumor.
Experimental evidence suggests that asbestos acts as a complete carcinogen with the development of mesothelioma occurring in sequential stages of initiation and promotion. The molecular mechanisms underlying the malignant transformation of normal mesothelial cells by asbestos fibers remain unclear despite the demonstration of its oncogenic capabilities (see next-but-one paragraph). However, complete in vitro transformation of normal human mesothelial cells to a malignant phenotype following exposure to asbestos fibers has not yet been achieved. In general, asbestos fibers are thought to act through direct physical interactions with the cells of the mesothelium in conjunction with indirect effects following interaction with inflammatory cells such as macrophages.
Intracellular
Analysis of the interactions between asbestos fibers and DNA has shown that phagocytosed fibers are able to make contact with chromosomes, often adhering to the chromatin fibers or becoming entangled within the chromosome. This contact between the asbestos fiber and the chromosomes or structural proteins of the spindle apparatus can induce complex abnormalities. The most common abnormality is monosomy of chromosome 22. Other frequent abnormalities include structural rearrangement of 1p, 3p, 9p and 6q chromosome arms.
Common gene abnormalities in mesothelioma cell lines include deletion of the tumor suppressor genes:
- Neurofibromatosis type 2 at 22q12
- P16INK4A
- P14ARF
Asbestos has also been shown to mediate the entry of foreign DNA into target cells. Incorporation of this foreign DNA may lead to mutations and oncogenesis by several possible mechanisms:
- Inactivation of tumor suppressor genes
- Activation of oncogenes
- Activation of proto-oncogenes due to incorporation of foreign DNA containing a promoter region
- Activation of DNA repair enzymes, which may be prone to error
- Activation of telomerase
- Prevention of apoptosis
Several genes are commonly mutated in mesothelioma, and may be prognostic factors. These include epidermal growth factor receptor (EGFR) and C-Met, receptor tyrosine kinases which are overexpressed in many mesotheliomas. Some association has been found with EGFR and epithelioid histology but no clear association has been found between EGFR overexpression and overall survival. Expression of AXL receptor tyrosine kinase is a negative prognostic factor. Expression of PDGFRB is a positive prognostic factor. In general, mesothelioma is characterized by loss of function in tumor suppressor genes, rather than by an overexpression or gain of function in oncogenes.
As an environmentally triggered malignancy, mesothelioma tumors have been found to be polyclonal in origin, by performing a X-inactivation based assay on epitheloid and biphasic tumors obtained from female patients. These results suggest that an environmental factor, most likely asbestos exposure, may damage and transform a group of cells in the tissue, resulting in a population of tumor cells that are, albeit only slightly, genetically different.
Immune system
Asbestos fibers have been shown to alter the function and secretory properties of macrophages, ultimately creating conditions which favour the development of mesothelioma. Following asbestos phagocytosis, macrophages generate increased amounts of hydroxyl radicals, which are normal by-products of cellular anaerobic metabolism. However, these free radicals are also known clastogenic (chromosome-breaking) and membrane-active agents thought to promote asbestos carcinogenicity. These oxidants can participate in the oncogenic process by directly and indirectly interacting with DNA, modifying membrane-associated cellular events, including oncogene activation and perturbation of cellular antioxidant defences.
Asbestos also may possess immunosuppressive properties. For example, chrysotile fibres have been shown to depress the in vitro proliferation of phytohemagglutinin-stimulated peripheral blood lymphocytes, suppress natural killer cell lysis and significantly reduce lymphokine-activated killer cell viability and recovery. Furthermore, genetic alterations in asbestos-activated macrophages may result in the release of potent mesothelial cell mitogens such as platelet-derived growth factor (PDGF) and transforming growth factor-β (TGF-β) which in turn, may induce the chronic stimulation and proliferation of mesothelial cells after injury by asbestos fibres
Asbestos also may possess immunosuppressive properties. For example, chrysotile fibres have been shown to depress the in vitro proliferation of phytohemagglutinin-stimulated peripheral blood lymphocytes, suppress natural killer cell lysis and significantly reduce lymphokine-activated killer cell viability and recovery. Furthermore, genetic alterations in asbestos-activated macrophages may result in the release of potent mesothelial cell mitogens such as platelet-derived growth factor (PDGF) and transforming growth factor-β (TGF-β) which in turn, may induce the chronic stimulation and proliferation of mesothelial cells after injury by asbestos fibres
The mechanisms of asbestos carcinogenicity are not fully understood. During the long latency period of mesothelioma, many pathogenentic events may occur that can contribute to MM. Compared to other cell types tested, human mesothelial cells are very susceptible to asbestos cytotoxicity. For example, when exposed to amosite asbestos, mesothelial cells were 10 and 100 times more sensitive to the cytotoxic effects of asbestos than normal human bronchial epithelial or fibroblastic cells. Asbestos fibers induce toxicity in a dose-dependent manner. In tissue culture, doses equal to or higher than 5 µg/cm2 of crocidolite fibers induce 100% cell death in less than a week. This observation raises the issue of how can asbestos cause mesothelioma if human mesothelial cells exposed to asbestos die. Recent work addressed this paradox and demonstrated a critical role for tumor necrosis factor-alpha (TNF-α) and NF-κB signaling in mediating responses of human mesothelial cells to asbestos. In vivo studies have revealed that, following asbestos exposure there is an inflammatory reaction with a large component of mononuclear phagocytes. Upon differentiation into macrophages, these cells phagocytize asbestos and, in response, release TNF-α. At the same time, asbestos induces human mesothelial cells to express TNF-α receptor TNF-R1 and also stimulates the secretion of TNF-α (both paracrine and autocrine effects). TNF-α binds to its receptor and activates the NF-κB pathway, which increases the percentage of human mesothelial cells that survive asbestos exposure. Human mesothelial cells exposed to asbestos can accumulate DNA damages. Asbestos causes DNA strand breaks mediated by iron-catalyzed free radicals. In addition, by causing the release of reactive oxygen species (ROS) and reactive nitrogen species (RNS), asbestos fibers can indirectly induce genotoxicity including base substitutions, deletions, rearrangements, insertions, sister chromatid exchanges, and chromosomal aberrations which may lead to a broad spectrum of mutations in mammalian cells. The activation of the NF-κB pathway stimulated by TNF-α allows mesothelial cells with asbestos-induced DNA damage to divide rather than die, and if sufficient specific genetic damage accumulates to eventually develop into a mesothelioma.
In addition to TNF-α, other growth factors and cytokines have been implicated in asbestos carcinogenesis and their role in MM pathogenesis is being investigated. These include: transforming growth factor beta (TGF-β), which might have a role in stimulating tumor growth; platelet-derived growth factor (PDGF), which may act as a regulatory factor in MM cell proliferation, insulin-like growth factor (IGF), which promotes tumor proliferation and cell migration; interleukins such as IL-6 and IL-8, which may promote tumor growth and the development of new capillaries; vascular endothelial growth factor (VEGF), which also promotes tumor angiogenesis, and hepatocyte growth factor (HGF), which stimulates mesothelioma cell migration and tumor invasiveness.
After interaction with mesothelial cells, asbestos triggers multiple cell-signaling pathways. Crocidolite fibers can induce autophosphorylation of the epidermal growth factor receptor, which stimulates the extracellular signal regulated kinase (ERK1/2) signaling pathway. This effect in turn increases activator protein (AP)-1 activity and mitosis of mesothelial cells. Asbestos also activates the NF-κB pathway, which leads to the activation of multiple pro-survival genes that promote tumor development.
Cytogenetic and loss of heterozygosity analyses of MMs have detected frequent deletions of specific regions within chromosome arms 1p, 3p, 4p, 4q, 6q, 9p, 13q, 14q, 15q and 22q. Certain tumor suppressor genes located in these chromosomal regions have also been implicated, including CDKN2A/ARF at chromosome band 9p21 and NF2 at 22q12. Mutations of the p53 gene (TP53) are occasionally observed in MMs. Loss and/or inactivation of these tumor suppressor genes may play a role in the development and progression of MM. For example, CDKN2A/ARF encodes the tumor suppressors p16(INK4a), a cyclin-dependent kinase inhibitor, and p14(ARF), a component of the p53 cell cycle checkpoint; and homozygous deletions of the CDKN2A/ARF locus in MM might simultaneously impair both the retinoblastoma (Rb) and p53 pathways. The NF2 product, Merlin, represses cyclin D1 expression, and loss/inactivation of NF2 in MM leads to cell cycle progression in connection with up regulation of cyclin D1. Merlin also inhibits Rac/Pak and focal adhesion kinase (FAK) signaling, which play a role in cell migration and spreading, and inactivation of NF2 in mesothelioma promotes cell invasiveness and spreading.
SV40
Simian virus 40 (SV40) is a DNA monkey virus that has been associated with MM. The most likely route of SV40 transmission from monkey to human was through the SV40 contaminated polio vaccines produced between 1955 and 1978.
Although over 50 different laboratories have reported a positive association of SV40 with mesothelioma, some have not, and this has caused a controversy. For example, Lopez-Rios et al. reported that initially they detected SV40 in about 60% of MM specimens, and then they determined that most of the positive results were caused by plasmid PCR contamination, and that only 6% of the initially positive samples were confirmed to contain SV40 DNA. It appears possible that some studies that lacked proper controls may have reported false positive results. However, many carefully designed and controlled studies have showed the presence of SV40 in human specimens by using several other techniques besides PCR, including Southern blotting, immunostaining, RNA in situ hybridization, microdissection, and electron microscopy. Analysis of human mesotheliomas revealed that SV40 sequences are present in tumor cells but not in the normal adjacent tissue. Animal experiments demonstrated a clear association between SV40 and MM. For instance, 100% of hamsters injected intrapleurally with SV40 and 60% of those injected intracardially developed mesothelioma within 6 months.
SV40 produces two proteins that are oncogenic: Large T and small t antigens. In human MM biopsies, the large T antigen (Tag) was found to bind and inhibit p53 and pRb tumor suppressor proteins, thus contributing to mesothelioma carcinogenesis. A recent study demonstrated that Tag-p53 complex also has growth stimulatory activities that are required for malignant cell growth. This investigation revealed that a multi-protein complex “Tag-p53-pRb-p300” binds to the promoter of the gene encoding insulin-like growth factor I (IGF-I), thereby stimulating IGF-I expression and IGF-I signaling and leading to enhanced cell growth. In other words, the binding of Tag to p53, p300, pRb, on one hand, inactivates the tumor suppressor activities of these proteins, on the other, the Tag-p53-pRb-p300 multi-protein complex acquire its own oncogenic activity by activating the IGF-1/IGF1R pathway. The small t antigen (tag) inhibits the cellular phosphatase 2A (PP2A), a protein involved in the dephosphorylation of many protein substrates, including components of the MAP-kinase (MAPK) pathway. Through inhibition of PP2A, tag may activate MAPK signaling and induce AP-1 activity. In addition, SV40 induces HGF/Met receptor activation, telomerase activity, and Notch-1 activation in human mesothelial cells and MM biopsies.
That SV40 and asbestos might be co-carcinogens was first demonstrated by Bocchetta et al. during in vitro studies of human mesothelial cells. These observations were confirmed by Kroczynska et al., who demonstrated a strong co-carcinogenic effect between asbestos and SV40. Asbestos and SV40 dl883 (SV40 dl883 does not express tag and it does not cause mesothelioma in animals) together caused mesothelioma in 90% of hamsters, whereas SV40 dl883 alone did not cause mesothelioma in any animal, and asbestos alone caused mesothelioma in only 20% of hamsters. Importantly, significantly lower amounts of asbestos were sufficient to cause MM in animals infected with SV40. Molecular studies showed that asbestos and SV40 in combination had a co-stimulatory effect in inducing ERK1/2 phosphorylation and AP-1 activity in both Syrian hamsters and human primary mesothelial cells. AP-1 activation stimulated the expression and activation of matrix metalloproteinases MMP-1 and MMP-9, which in turn led to cell invasion. These findings indicate that mineral fibers and viruses can act as co-carcinogens. Moreover, these data suggest that lower amounts of asbestos may be sufficient to cause mesothelioma in individuals infected with SV40. These results are important for determining levels of asbestos exposure that are supposedly “safe”. Such supposedly safe levels may not be truly safe for the millions of individuals who were exposed to SV40 contaminated polio vaccines. Co-carcinogenesis between SV40 and asbestos was later confirmed by Robinson et al and Pietruska et al in different animal models.
Genetics
Genetic susceptibility to mesothelioma was observed in the Cappadocian villages of Tuzkoy, Karain, and “Old” Sarihidir. Although mineralogical studies showed that all the houses appear to contain similar amounts of erionite, mesothelioma was prevalent in certain families but not in others. Pedigree studies of the three mesothelioma villages showed that it seemed to be inherited in an autosomal dominant pattern. When high-risk mesothelioma family members married into families with no history of the disease, mesothelioma developed in the descendants. Taken together, the results of mineralogical studies and pedigree analysis indicate that the epidemic in Cappadocia is caused by erionite exposure in genetically predisposed individuals.
 |
| Example of genetic familial mesothelioma of a family pedigree found in a village in Turkey : showing a family of 30 members in which 17 died of mesothelioma (black symbols), 4 died of other cancers [osteosarcomas (B), leukemia (D), prostate cancer (F), and pancreatic cancer (G)], 4 died of reasons other than cancer [2 traffic accidents (A), 1 intestinal occlusion (C), 1 congestive heart failure (E), and 1 unknown reason (first generation, female; F)], and 4 are alive (white symbols). Five mesothelioma developed in individuals who married into the family. They were also from mesothelioma families. Bottom, representative examples. Family 9: Pedigree of the family of origin of 65-year-old male (+) who married into family 1. Seven of the 17 people of this two-generation pedigree died of mesothelioma, 1 of liver cancer (H), and 5 unknown. The deaths from mesothelioma include a 46-year-old female (^) who married into family 9. The family of origin of this woman (family 10) has a very high incidence of mesothelioma: 5 of the 7 family members died of mesothelioma and 1 of lung cancer (K); the remaining cause of death is unknown. Family 3: When members of family 1 (*) marry into a non– mesothelioma family, the cancer appeared in the descendents. A, traffic accident; C, intestinal occlusion; the other causes of death were not cancer-related, but they could not be established with certainty. *MM=mesothelioma. |
Genetically predisposed family members born and raised outside the villages did not seem to develop mesothelioma, supporting the observation that the combination of genetics and erionite exposure (gene and environment) is involved in causing mesothelioma in these villages. Several US families have incidences of mesothelioma similar to those found in the Cappadocian families. It is possible that in the US mesothelioma families, genetic predisposition and asbestos exposure (or SV40) cause mesothelioma. However, the largest deposits of erionite are in the US, therefore, there is the possibility that erionite also played a role in these or other US mesothelioma.
SIGNS AND SYMPTOMS OF ASBESTOSIS (CLINICAL MANIFESTATIONS)
Symptoms and signs of asbestosis can include (The effects of long-term exposure to asbestos typically don't show up for 10 to 40 years after initial exposure) :
- shortness of breath
- persistent dry cough
- wheezing / crackling sound when breathing
- chest tightness
- hypertension (high blood pressure)
- loss of appetite with weight loss
- extreme tiredness (fatigue)
- swelling in the neck or face
- difficulty swallowing
- blood in sputum
- pain in your chest or shoulder
- nail deformities
- in more advanced cases, clubbed (swollen) fingertips / finger deformity
Asbestosis is a type of pulmonary fibrosis caused by asbestos exposure typified by excess connective tissue in the lungs. Because the disease manifests in the lungs, common asbestosis symptoms include respiratory problems such as coughing, swelling in the neck or face, cracking sound when breathing, or difficulty swallowing.
Fibrosis usually occurs due to the lungs reacting to and repairing damage to lung tissue over a long period of time; such as, continuous exposure to asbestos fibers. This reparative scar tissue replaces normal lung tissue, and an excess amount of scar tissue can cause reduced pulmonary function.
During exposure, asbestos fibers are inhaled, and they can become lodged in lung tissue. The sharp, straight shape of the fibers makes them difficult for a body to dislodge and expel. Once in a body for a long period, the fibers cause irritation, inflammation and scarring, which cause symptoms that primarily affect the lungs.
In most asbestosis patients, symptoms develop within 20 to 30 years after being exposed to asbestos. If someone is exposed to asbestos for a long time, a decade or more, the latency period of symptom development is shorter: closer to 20 years.
Symptoms of asbestosis generally consist of respiratory problems. The two most common symptoms are coughing and shortness of breath, followed by chest pain and clubbing of the fingers. In the early stages of the disease, people experience shortness of breath and fatigue during physical activity. As the condition progresses, being short of breath even while doing very little becomes an issue. This is usually enough to encourage someone with asbestosis to take the first step in the diagnostic process and schedule an appointment with their primary care physician.
Lung scarring, or fibrosis, is the direct cause for the coughing and shortness of breath symptoms most commonly associated with asbestosis.
As the lungs become scarred and inflamed over time, their ability to exchange oxygen and carbon dioxide decreases, resulting in a reduction of lung function and subsequent fatigue in patients. In the later stages of asbestosis, the amount of stress placed on the lungs and heart from the lack of proper oxygen can lead to serious lung and/or heart failure.
Shortness of breath arises because of pleural thickening, the thickening of the lining of the lungs, caused by the longtime presence of asbestos fibers, or pleural effusion, the buildup of fluid between the chest wall and the lungs. Effusions can be caused by many conditions (pneumonia, lupus, congestive heart failure) and can stem from inflammation of the lungs. The thickening and effusions constrict movement of the lungs and eventually the heart. At that point, neither organ expands or contracts properly, which leads to shortness of breath and more fluid build up.
Asbestosis can set in motion a cycle of conditions. The disease prevents lungs from fully oxygenating blood, forcing the heart to work harder. As the heart works harder, blood pressure increases. As blood pressure increases, fluid builds up around the heart and lungs, which can lead to swelling in the neck and face, which in turns can lead to difficulty swallowing.
Fluid up can also build up in the abdomen, creating bloating or tenderness, which can lead to a loss of appetite and potential weight loss. In advanced cases, fluid retention, if untreated, will lead to finger deformity, known as clubbing.
Dyspnea (shortness of breath) upon exertion is the most common symptom and worsens as the disease progresses. Patients may have a dry (ie, nonproductive) cough. A productive cough suggests concomitant bronchitis or a respiratory infection. Patients may report nonspecific chest discomfort, especially in advanced cases.
DIAGNOSIS OF ASBESTOSIS
Asbestosis is usually diagnosed by a careful medical history, exposure history and chest X-ray or CT scan that shows scarring of the lung tissues. This information, along with breathing tests, will help to determine how severe the asbestosis is and how much of the lung is working.
Diagnosing asbestosis is not simple, and it can mean a number of visits to the doctor and a variety of tests. It’s possible or even likely that a primary care physician may not be able to diagnose or detect asbestosis because its early symptoms are like those of so many other conditions. For this reason, patients may be sent to an oncologist or pulmonologist for additional testing. An oncologist or pulmonologist can determine which tests or imaging scans are needed and how to evaluate each of them.
An oncologist or pulmonologist are also more familiar with the criteria for confirming a diagnosis of asbestosis and ruling out other asbestos-related diseases like mesothelioma or lung cancer. Before asbestosis can be considered as a probable cause of symptoms, several indicators must be present, including a history of asbestos exposure, a latency period between the exposure and the onset of symptoms, evidence of structural changes to the lungs and evidence of cause.
A complete medical evaluation is needed before a proper asbestosis diagnosis can be made. This includes reviewing potential asbestos exposure, work history, symptoms and undergoing various tests and imaging scans that can detect lung abnormalities. Most cases are diagnosed late because symptoms do not develop until the condition has reached a more developed stage. However, early detection is possible if those previously exposed to asbestos receive annual medical exams that check for asbestos-related disease.
Asbestos Exposure History
For someone to be at risk for developing asbestosis, there has to be a time during their life of consistent and extended exposure to asbestos, often for more than five years. All sources of asbestos exposure should be discussed during the evaluation and if possible, identify the duration, intensity and circumstance under which the exposure occurred.
Any history of asbestos exposure should start with a work history. Most people diagnosed with asbestos-related diseases, including asbestosis, come into contact with the mineral while on the job, so a job history is an important factor. Previous occupations should be questioned.
There is no such thing as an occupation that is most at risk, but several of them are: welders, floor installers, drywall installers, shipyard workers, miners, insulators, roofers, and auto mechanics. In addition, those who served in the military are considered high-risk for developing an asbestos-related disease.
Latency Period
Latency – the amount of time between excessive exposure to asbestos and the time someone is confirmed to have the disease – is an integral part of the diagnostic process. If fibrosis is detected within only a few years since exposure, doctors are likely to look at other lung diseases before confirming asbestosis. Depending on the level of exposure, asbestosis usually takes 20 years or more from the time of initial exposure to present symptoms. Although clinical signs of asbestosis development can be seen in imaging scans as early as five years after exposure, such early detection is rare.
Evidence of Structural Physical Changes
An asbestosis diagnosis cannot be confirmed until imaging scans demonstrate structural changes to the lungs. Signs of pleural thickening, pleural effusion and scarring are all indicators that asbestosis may be present.
Evidence of Etiology by Asbestos
Even though asbestos exposure may have occurred, it does not mean someone will develop lung problems as a result. Having occupational or environmental history as a probable source for exposure is necessary before asbestosis is even considered. In addition, the presence of pleural plaques or asbestos fibers in the lung tissue is the kind of evidence that often leads to an absolute diagnosis.
To accurately diagnose and confirm asbestosis, one or more tests may be required. Evaluating a series of imaging scans is the most common technique used to diagnose asbestosis.
Anamnesis
During the visit, the patient should be asked about breathing, both at rest and during exercise. The patient should also be asked about jobs in detail to determine how much the patient were exposed to asbestos. A few helpful points to ask a patient :
- The symptoms and the time they started
- Treatments given before for the symptoms
- The work that has been done in the patient’s entire career; the length of time spent in each job
- The products the patient were in contact with at work and whether or not they wore protective equipment
- Smoking history
- Any old medical records, including chest X-rays or CT scans
Physical examintion
One of the first tools a physician will use in the diagnostic process is a stethoscope, which allows them to listen to the lungs. If the lungs are affected, a doctor will likely hear a dry, crackling sound while the patient breathes.
Rales (an abnormal crackling or rattling sound heard upon auscultation of the chest) are the most important finding during examination. Persistent and dry, they are described as fine cellophane rales or coarse Velcro rales. The rales are best auscultated at the bases of the lungs posteriorly and in the lower lateral areas.
Initially, physicians hear rales in the end-inspiratory phase. In advanced disease, however, rales may be heard during the entire inspiratory phase. Occasionally, the presence of rales precedes radiographic finding abnormalities and pulmonary function test abnormalities. Rales are not to be expected in all patients; one third of them may not have this symptom.
Finger clubbing is observed in 32-42% of cases. This finding is not necessarily related to the severity of disease.
Reduced chest expansion in advanced disease correlates with restrictive ventilatory impairment and reduced vital capacity. In advanced disease, patients may show the following signs associated with cor pulmonale: cyanosis, jugular venous distention, hepatojugular reflux, and pedal edema.
Diagnostic tests
Asbestosis can be difficult to diagnose because its signs and symptoms are similar to those of many other types of respiratory diseases. A variety of diagnostic tests might be needed to help pinpoint the diagnosis.
After the preliminary physical examination, doctors will perform a range of tests and imaging scans before an asbestosis diagnosis can be confirmed. Tests for asbestosis will analyze lung function or collect a lung sample for lab testing. A series of imaging scans are also required to detect locations where asbestos fibers have caused scarring of the lungs. While evaluating the results of these imaging scans, an oncologist will look for dark spots or pleural thickening as signs of asbestosis.
Blood tests for antinuclear antibodies (ANAs), rheumatoid factor, and erythrocyte sedimentation rate lack diagnostic specificity and are not useful in diagnosis or in activity assessment.
A lung scan with gallium citrate (67 Ga) is a nonspecific test that may detect areas of inflammation in the lungs. However, the results do not always correlate with other measurements of inflammation. This test is no longer recommended.
Biopsy, where small samples of lung tissue are surgically removed and then examined for the scars and tiny asbestos fibers, is usually not necessary to diagnose asbestosis.
Physicians often make the diagnosis without histopathologic confirmation. Errors may occur because other, more common interstitial diseases (eg, idiopathic pulmonary fibrosis) mimic the clinical, radiologic, and pulmonary functional features of asbestosis. Bear in mind the long latency period that exists between patient exposure and the manifestation of symptoms and signs of asbestosis.
When lung tissue is available for histopathologic examination, confirmation of diagnosis requires both fibrosis and accumulation of fibers or asbestos bodies (ie, ferruginous bodies; these are asbestos fibers that develop a ferritin-protein coat and have a characteristic long-beaded appearance). Asbestos bodies alone are not diagnostic for disease, because occasionally examiners find asbestos bodies in people without known exposure.
Pleural plaques may coexist with asbestosis, but these plaques alone are usually not associated with impaired pulmonary function. Nonetheless, pleural plaques are a reliable indicator of asbestos exposure.
Imaging tests
Chest X-ray
The abnormal chest x-ray and its interpretation remain the most important factors in establishing the presence of pulmonary fibrosis. The findings usually appear as small, irregular parenchymal opacities, primarily in the lung bases. Using the ILO Classification system, "s", "t", and/or "u" opacities predominate.
A chest X-ray is used to detect any abnormalities present in lung tissue. On an X-ray, scarred lung tissue developing from asbestos exposure appears as dark, opaque areas, meaning light has a difficult time passing through it. In advanced cases of asbestosis in which an entire lung is affected, the area may have a honeycomb-like appearance in the upper lobe. Bilateral interstitial fibrosis, meaning both lungs exhibit fibrosis, may also be present.
More than 50% of people affected with asbestosis develop plaques in the parietal pleura, the space between the chest wall and lungs. Once apparent, the radiographic findings in asbestosis may slowly progress or remain static, even in the absence of further asbestos exposure. Rapid progression suggests an alternative diagnosis.
Advanced asbestosis appears as excessive whiteness in the lung tissue. If the asbestosis is severe, the tissue in both lungs might be affected, giving them a honeycomb appearance.
Chest radiographs (ie, posteroanterior and lateral views) are basic and required diagnostic imaging studies. However, the diagnosis of asbestosis requires multiple elements. A chest radiograph alone has only a modest positive predictive value for the condition, but when it is combined with abnormal signs (rales) and pulmonary function test results, the positive predictive value is markedly increased.
Typical findings include diffuse reticulonodular infiltrates, which are observed predominantly at the lung bases. The diffuse lung infiltrates cause the appearance of shaggy heart borders.
In early disease, an increase in interstitial markings, mostly linear, is seen. Honeycombing, with cystic spaces surrounded by coarse interstitial infiltrates and small lung fields, characterizes advanced disease.
Bilateral pleural thickening may be observed. Asbestos-related pleural thickening more often involves the middle third of the pleura as opposed to the upper third, which is affected by tuberculosis, or the lower third, which can be damaged by empyema, trauma, or past pleurodesis therapy. (An oblique-view radiograph may be helpful in recognizing pleura-based abnormalities.)
A calcified pleural plaque located in the diaphragmatic pleura is a reliable indicator of asbestos exposure but is not a required element for the diagnosis of asbestosis. Besides the diaphragmatic pleura, other common sites for plaque formation in the parietal pleura are along the sixth through the ninth ribs. Noncalcified plaques may not be detected on chest radiographs.
Computerized tomography (CT).
A computerized tomography (CT) scan may be recommended to confirm a diagnosis of asbestosis. A CT scan can also be used for screening purposes, as these scans can sometimes detect asbestosis sooner than chest X-rays. In most cases though, a CT scan is used when doctors cannot get a confirmation from a chest radiograph.
Computed tomography (CT) scanning is also useful in the delineation of pleural or pleura-based abnormalities (eg, effusion, thickening, plaque, malignant mesothelioma, rounded atelectasis) and in the delineation of a parenchymal density that is suggestive of bronchogenic carcinoma. CT or high-resolution CT (HRCT) are more sensitive than plain radiography at detecting pulmonary fibrosis (as well as any underlying pleural changes).
CT scans combine a series of X-ray views taken from many different angles to produce cross-sectional images of the bones and soft tissues inside the patients body. These scans generally provide greater detail and might help detect asbestosis in its early stages, even before it shows up on a chest X-ray.
A high-resolution CT (HRCT) scan allows better definition of interstitial infiltrates and may be helpful in diagnosing asbestosis in the early stages.
Typical HRCT findings in asbestosis include subpleural linear opacities seen parallel to the pleura; basilar lung fibrosis and peribronchiolar, intralobular, and interlobular septal fibrosis; honeycombing; and pleural plaques.
In a minority of cases, HRCT abnormalities may be seen in individuals with normal chest radiographic findings.
Pulmonary (lung) function tests
Pulmonary Function Test: This test, which can be used for people suspected of having asbestosis, determines how well lungs are functioning. It is designed to test the air capacity of the lungs (how much air they can hold) and determine how well air flow is going in and out. The most common way of measuring these functions is by blowing into an instrument called a spirometer. Some reports have indicated the occurrence of abnormal pulmonary function tests in 50 to 60 percent of asbestosis patients. One of the key signs of the disease is a reduction in forced vital capacity, or the most air a patient can force from their lungs after a full inhalation.
Spirometer
These tests determine how well the patients lungs are functioning. Pulmonary function tests measure how much air the lungs can hold and the airflow in and out of your lungs.
During the test, the patient will be asked to blow as hard as he or she can into an air-measurement device called a spirometer. More-complete pulmonary function tests can measure the amount of oxygen being transferred to the bloodstream.
Diffusing capacity reduction precedes lung volume changes, but findings from a diffusing capacity measurement are not specific. Besides diffusing capacity reduction, the earliest physiologic abnormality is exertional hypoxemia. Total lung capacity is reduced in asbestosis as in other restrictive disorders.
Using spirometry, vital capacity on a pulmonary function test typically appears reduced, without a reduction in the ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1 to FVC).
Small-airway flow rates (eg, midexpiratory forced expiratory flow [FEF25-75]) are reduced but are nonspecific for a diagnosis of small-airway obstructive disease.
The evaluation of oxygenation is important because uncorrected hypoxemia causes pulmonary hypertension and may lead to cor pulmonale.
Physicians can use a noninvasive test of pulse oximetry as a screening test, especially if oximetry is performed during rest and during exercise (eg, 6-minute walk test).
Obtain accurate information through measurement of arterial blood gases, which requires an arterial puncture. In selected cases, an exercise study may demonstrate desaturation during exercise.
Bronchoscopy
This procedure is where a thin fiber-optic scope is inserted into the lungs. The procedure is performed to obtain fluid or tissue samples from the lung area. A patient whose diagnosis is difficult to confirm may undergo this procedure so that fluid and tissue samples can be tested to ensure a correct diagnosis.Fiberoptic bronchoscopy is performed to facilitate BAL. In addition, bronchoscopy is indicated for airway examination when radiologic studies are suggestive of bronchogenic carcinoma. Transbronchoscopic lung biopsy is not recommended for diagnosis of asbestosis. This procedure yields inadequate tissue and may cause crush alterations to the tissue.
Bronchoalveolar lavage
Bronchoalveolar lavage (BAL) has only limited application in the diagnosis and management of asbestosis. BAL is helpful in diagnosing infections that may present with diffuse infiltrates and simulate asbestosis, and the procedure may aid in the diagnosis of a coexisting bronchogenic carcinoma. In workers who are exposed to asbestos, BAL can provide quantitative information through asbestos fiber counts. More than 1 asbestos body (ie, coated asbestos fiber) per milliliter of lavage effluent suggests significant exposure.
Histologic Findings
In most cases, physicians diagnose asbestosis without a histopathologic examination of lung tissue. Asbestos bodies (ie, ferruginous bodies) are asbestos fibers that develop a ferritin-protein coat and have a characteristic long-beaded appearance. Asbestos bodies alone are not diagnostic for disease, because occasionally examiners find asbestos bodies in people without known exposure. The presence of pleural plaquing may provide supportive evidence of causation by asbestos. Conversely, interstitial pulmonary fibrosis in the absence of asbestos bodies is most likely not asbestosis. Asbestos bodies in the absence of fibrosis indicate exposure, not disease. A pathologic diagnosis of asbestosis requires visualization of both fibrosis and asbestos bodies through light microscopy or a significant quantity of asbestos fibers observed through electron microscopy.
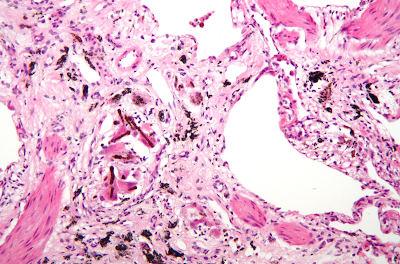 |
| A high magnification micrograph with a hematoxylin and eosin stain coloring of a lung biopsy of an asbestosis patient, showing characteristic ferruginous bodies and interstitial fibrosis. |
Histologic Grades of Asbestosis
Grade of Severity
- Grade 0 : Early fibrosis involving walls of at least one respiratory bronchiole, with or without extension into nearby tissue. Fibrosis is confined to alveolated walls of respiratory bronchioles and ducts and not present in more distant alveoli. Inflammation similar to that caused by cigarette smoking may be observed.
- Grade 1 : Early fibrosis involving walls of at least one respiratory bronchiole, with or without extension into nearby tissue. Fibrosis is confined to alveolated walls of respiratory bronchioles and ducts and not present in more distant alveoli. Inflammation similar to that caused by cigarette smoking may be observed.
- Grade 2 : More severe fibrosis involving alveolar ducts and/or two or more layers of adjacent alveoli. Normal lung tissue remains in an area between adjacent bronchioles.
- Grade 3 : Fibrosis is advanced and involves alveolar ducts and all layers of adjacent alveoli. All lung tissue between at least two adjacent bronchioles is affected. Some alveoli are completely damaged.
- Grade 4 : Honeycomb-like appearance and large (up to 1 cm) dilated spaces largely visible in lung parenchyma, or the alveolar tissue.
Grade of Extent
- Grade A : Only occasional bronchioles are involved. Most appear normal.
- Grade B : More than occasional but less than half of bronchioles are involved.
- Grade C : More than half of bronchioles are involved.
According to the American Thoracic Society (ATS), the general diagnostic criteria for asbestosis are:
- Evidence of structural pathology consistent with asbestosis, as documented by imaging or histology
- Evidence of causation by asbestos as documented by the occupational and environmental history, markers of exposure (usually pleural plaques), recovery of asbestos bodies, or other means
- Exclusion of alternative plausible causes for the findings
Asbestosis resembles many other diffuse interstitial lung diseases, including other pneumoconiosis. Although lung biopsy is usually not necessary, the presence of asbestos bodies in association with pulmonary fibrosis establishes the diagnosis. The differential diagnosis of asbestosis includes idiopathic pulmonary fibrosis (IPF), hypersensitivity pneumonitis, sarcoidosis, and others.
SIGNS AND SYMPTOMS OF MESOTHELIOMA (CLINICAL MANIFESTATIONS)
Pleural mesothelioma
Anywhere from 20 to 50 years (or more) can pass between the time a person is exposed to asbestos and when pleural mesothelioma symptoms begin to appear. Once symptoms do begin to occur, they often show up first in the chest and respiratory system, although some symptoms (like weight loss or fever) can be systemic. Shortness of breath, cough, and pain in the chest due to an accumulation of fluid in the pleural space (pleural effusion) are often symptoms of pleural mesothelioma.
The initial symptoms of pleural mesothelioma include chest pain, shortness of breath, slight fatigue and weight loss. Because these symptoms mirror those of less serious illnesses, such as pneumonia or the flu, doctors often misdiagnose the cancer in its early stages.
Unfortunately, many of the more serious symptoms, such as painful breathing, coughing blood and difficulty swallowing, aren’t noticeable until the cancer has reached its later stages, when treatment options are usually more limited.
Mesothelioma that affects the pleura can cause these signs and symptoms:
- Fatigue or anemia
- Chest wall pain, Pain in the lower back or side of the chest
- Pleural effusion, or fluid surrounding the lung
- Shortness of breath
- Wheezing, hoarseness, or a dry cough
- Difficulty swallowing
- Blood in the sputum (fluid) coughed up (hemoptysis)
Less common symptoms :
- Weight loss
- Fever
- Night sweats
- Swelling in the face and arms
In severe cases, the person may have many tumor masses. The individual may develop a pneumothorax, or collapse of the lung. The disease may metastasize, or spread to other parts of the body.
Pleural mesothelioma can also be accompanied by a set of other conditions that could display symptoms of their own. These include:
- Pleural plaque – a chalky substance that forms on the lungs due to calcification
- Diffuse pleural thickening (DPT) – Gray, fibrous tissue that fills in pleural spaces
- Asbestosis – Scarring of the lungs (fibrosis)
These conditions may also occur on their own in individuals who do not have pleural mesothelioma.
Peritoneal mesothelioma
Due to the location of the disease, peritoneal mesothelioma symptoms most often develop in the abdomen and/or gastrointestinal system, rather than the chest and lungs.
The most common symptoms of peritoneal mesothelioma are abdominal swelling and pain due to ascites (a buildup of fluid in the abdominal cavity). Other features may include weight loss, fever, night sweats, poor appetite, vomiting, constipation, and umbilical hernia. If the cancer has spread beyond the mesothelium to other parts of the body, symptoms may include pain,, trouble swallowing, or swelling of the neck or face. These symptoms may be caused by mesothelioma or by other, less serious conditions.
Tumors that affect the abdominal cavity often do not cause symptoms until they are at a late stage. Symptoms include:
- Abdominal pain
- Ascites, or an abnormal buildup of fluid in the abdomen
- A mass in the abdomen
- Problems with bowel function
- Weight loss
Pericardial mesothelioma
As this disease affects the heart, the main symptoms affect cardiovascular operation, including chest pain, fluid buildup in the heart (pericardial effusion), heart murmurs, arrhythmia, and pulse variance.
Pericardial mesothelioma is not well characterized, but observed cases have included cardiac symptoms, specifically constrictive pericarditis, heart failure, pulmonary embolism, and cardiac tamponade. They have also included nonspecific symptoms, including substernal chest pain, orthopnea (shortness of breath when lying flat), and cough. These symptoms are caused by the tumor encasing or infiltrating the heart.
End stage mesothelioma
In severe cases of the disease, the following signs and symptoms may be present:
- Blood clots in the veins, which may cause thrombophlebitis
- Disseminated intravascular coagulation, a disorder causing severe bleeding in many body organs
- Jaundice, or yellowing of the eyes and skin
- Low blood sugar level
- Pleural effusion
- Pulmonary emboli, or blood clots in the arteries of the lungs
- Severe ascites
If a mesothelioma forms metastases, these most commonly involve the liver, adrenal gland, kidney, or other lung
Staging of mesothelioma
Staging procedures
The optimal preoperative staging procedures are debatable. In 1996, Sugarbaker et al recommend MRI as a standard part of staging. Others argue that laparoscopic thoracoscopy is the best way to determine the extent of the disease. Some argue that PET scans may be helpful, but their role in staging needs to be defined.
MRI performed with different pulse sequences and gadolinium-based contrast material can improve detection of tumor extension, especially to the chest wall and diaphragm. PET scans can provide metabolic and anatomic information, especially for patients with extrathoracic or mediastinal metastasis. The appropriate role of PET scans in the management of malignant mesothelioma is still undefined.
In 1996, Sugarbaker and associates proposed the Brigham staging system based on tumor resectability and nodal status, a system validated in a clinical trial. To date, the accepted system is the TNM classification accepted by the International Mesothelioma Interest Group (IMIG). The stages of mesothelioma are as follows:Upon diagnosis, the doctor will categorize the disease into one of four stages. While there are several staging systems, the TNM System — which stands for tumor, lymph nodes, and metastasis — is the most commonly used. Which can be summarized as :
- Stage I: This stage is divided into two categories. During stage 1a, the cancer is localized to the outer layer of the pleura, which is closer to the chest wall. At stage 1b, the cancer is also located on the inner layer of the pleura, which is closer to the lung. Or in medical terms, completely resected within the capsule of the parietal pleura without adenopathy (ie, ipsilateral pleura, lung, pericardium, diaphragm, or chest wall disease limited to previous biopsy sites)
- Stage II: All stage I characteristics, with positive resection margins, intrapleural adenopathy, or a combination. The cancer has spread to the lung tissue, diaphragm and linings of the chest cavity.
- Stage III: Local extension of disease into the chest wall or mediastinum, into the heart, through the diaphragm or peritoneum, or extrapleurally to involve the lymph nodes. The cancer has advanced beyond the lining of the lungs and impacted other internal organs, lymph nodes near the main tumor, esophagus, trachea, fatty tissues and possibly other nearby areas.
- Stage IV: Distant metastatic (spread) disease. The cancer is possibly on both sides of the chest cavity, inside distant lymph nodes and in other organs such as the brain, spine and prostate. At stage IV, pleural mesothelioma cancer cannot be treated with surgery because metastasis (the spread of the cancer) is too extensive.
DIAGNOSIS OF MESOTHELIOMA
To ensure a definitive diagnosis, the doctor should first conduct a full medical and occupational history review. Then you will typically undergo multiple imaging tests such as X-rays, CT scans or PET scans.
One of the important step of the diagnostic process is the biopsy, in which a surgeon collects samples of the tumor through a minor outpatient surgical procedure known as a thoracoscopy or video-assisted thoracoscopic surgery (VATS). A pathologist then analyzes the samples to determine what kind of disease or cancer is present.
- Step 1: Body scans (X-ray, CT, PET or MRI)
- Step 2: Thoracentesis, Needle biopsy, Open surgical biopsy or video-assisted thoracic surgery
- Optional Step: Blood tests using biomarkers
Diagnosis of mesothelioma can be suspected with imaging but is confirmed with biopsy. It must be clinically and histologically differentiated from other pleural and pulmonary malignancies, including reactive pleural disease, primary lung carcinoma, pleural metastases of other cancers, and other primary pleural cancers. Primary pericardial mesothelioma is often diagnosed after it has metastasized to lymph nodes or the lungs.
Malignant pleural mesothelioma symptoms often present with symptoms that are similar to those of other diseases, making diagnosis extremely difficult in many cases. The most common way to diagnose the disease is to undergo a series of tests that can rule out other diseases, including various types of cancer.
The first step is usually to perform one or more imaging scans (x-ray, CT, PET, or MRI) to identify potential tumors. If such a tumor is detected, one or more blood tests may be performed to look for certain biomarkers (high levels of specific substances in the blood). If these tests point toward the possibility of mesothelioma, the diagnosis will need to be verified through a biopsy – usually through a thoracoscopy, thoracotomy, thoracentesis, or mediastinoscopy.
The most common misdiagnoses for pleural mesothelioma include:
- Chronic Obstructive Pulmonary Disease (COPD)
- Pneumonia
- Asthma
- Influenza (the flu)
- Other chest cancers, such as lung cancer or adenocarcinoma
Malignant mesothelioma is a difficult diagnosis to establish, so the pathologist should be warned if the index of suspicion is high. The diagnosis could be work related, and a thorough discussion with the patient is warranted.
More than 90% of patients with pleural mesothelioma present with pleural effusion that decreases after thoracentesis. Cytologic examination findings are diagnostic in only 32% of patients and are suggestive in 56% of patients. Thoracoscopically guided biopsy should be performed if mesothelioma is suggested; the results are diagnostic in 98% of cases.
Careful scrutiny of routinely stained biopsy preparations is the most valuable diagnostic tool in malignant mesothelioma. A battery of commercial immunohistochemistry stains (eg, for cytokeratins, vimentin, human milk fat globulin-2, anti-Leu M1, BerEP4, carcinoembryonic antigen) can be used.
Diagnostic features distinguishing malignant mesothelioma from adenocarcinoma include negative test results for periodic acid-Schiff stain, mucicarmine stain, carcinoembryonic antigen, and Leu M1 and positive test results for calretinin, vimentin, and cytokeratin. Electron microscopy reveals that cells have long microvilli, in contrast to adenocarcinomas, which have short microvilli.
One of the new most intriguing markers is serum mesothelin-related protein (SMRP), measured in fluid or serum. The circulating SMRP level has been reported to be elevated in 84% of patients with malignant mesothelioma and in 2% of patients with lung cancer.
Determining the extent of disease by performing a laparoscopy or magnetic resonance imaging (MRI) scan and a cardiopulmonary evaluation is important, if the patient is amenable.
Imaging tests
Diagnosing mesothelioma is often difficult because the symptoms are similar to those of a number of other conditions. Diagnosis begins with a review of the patient's medical history. A history of exposure to asbestos may increase clinical suspicion for mesothelioma. A physical examination is performed, followed by chest X-ray and often lung function tests. The X-ray may reveal pleural thickening commonly seen after asbestos exposure and increases suspicion of mesothelioma. A CT (or CAT) scan or an MRI is usually performed. If a large amount of fluid is present, abnormal cells may be detected by cytopathology if this fluid is aspirated with a syringe. For pleural fluid, this is done by thoracentesis or tube thoracostomy (chest tube); for ascites, with paracentesis or ascitic drain; and for pericardial effusion with pericardiocentesis. While absence of malignant cells on cytology does not completely exclude mesothelioma, it makes it much more unlikely, especially if an alternative diagnosis can be made (e.g. tuberculosis, heart failure). However, with primary pericardial mesothelioma, pericardial fluid may not contain malignant cells and a tissue biopsy is more useful in diagnosis. Using conventional cytology diagnosis of malignant mesothelioma is difficult, but immunohistochemistry has greatly enhanced the accuracy of cytology.
Chest radiographs in malignant pleural mesothelioma show obliteration of the diaphragm; nodular thickening of the pleura; decreased size of the involved chest; radiolucent, sheetlike encasement of the pleura; or a combination of these. A loculated effusion is present in more than 50% of patients, and a major portion of the pleura is opacified by the effusion.
A computed tomography (CT) or MRI scan of the chest or a positron emission tomography (PET) scan can also be used in the diagnosis of mesothelioma. However, the PET scan is still considered investigational for helping to differentiate between benign and malignant mesothelioma.
A CT-scan (Computerized Tomography) test is used more towards determining the stage of the tumor, while an MRI (Magnetic Resonance Imaging) in some patients is performed as a comparison with the CT readings, by showing better enhanced imaging of the soft tissue (better soft tissue contrast) and being able to provide imaging on the sagittal and coronal plane (section).
A PET (Positron Emission Tomography) reading could be useful to determine the boundaries of the spreading tumor or metastasis.
In a study looking at the value of fluorodeoxyglucose PET (FDG-PET) scanning in 17 patients with pleural mesothelioma, the survival period in the group with high FDG uptake was shorter than that in the low FDG group.
Biopsy
Generally, a biopsy is needed to confirm a diagnosis of malignant mesothelioma. A doctor removes a sample of tissue for examination under a microscope by a pathologist. A biopsy may be done in different ways, depending on where the abnormal area is located. If the cancer is in the chest, the doctor may perform a thoracoscopy. In this procedure, the doctor makes a small cut through the chest wall and puts a thin, lighted tube called a thoracoscope into the chest between two ribs. Thoracoscopy allows the doctor to look inside the chest and obtain tissue samples. Alternatively, the chest surgeon might directly open the chest (thoracotomy). If the cancer is in the abdomen, the doctor may perform a laparoscopy. To obtain tissue for examination, the doctor makes a small incision in the abdomen and inserts a special instrument into the abdominal cavity. If these procedures do not yield enough tissue, an open surgical procedure may be necessary.
Laboratory Studies/Tests
Blood samples or other types of test samples such as fluid, tissue or urine are examined in a lab to find evidences that may indicate cancer. The samples can reveal proteins, cancer cells or other substances that may indicate the presence of cancer. Results obtained through blood and urine tests can also help the doctor to check if your organs are functioning properly or if the tumor has started affecting them.
Explained below are some of the commonly used blood and urine tests:
- Complete Blood Count (CBC): This test is used for measuring the total amount of different types of blood cells as might be present in a blood sample taken from your body. It may detect blood cancers if results indicate too few or too many of a particular type of blood cell or if abnormal cells have been found. The diagnosis of blood cancer is confirmed through a bone marrow biopsy.
- Urine cytology: A microscopic analysis of urine sample can reveal the presence of cancer cells that may be originating from the kidneys, ureters, or the bladder.
- Blood protein testing: This test involves analysis of different types of proteins present in your blood (electrophoresis). It can be used to detect certain abnormal proteins in the immune system (immunoglobulins) that sometimes display an elevated presence in individuals with multiple myeloma. Some other tests, including the bone marrow biopsy, are done to confirm the suspected presence of cancer.
- Tumor marker tests: Chemicals produced by tumor cells, which can be identified in your blood are called tumor markers. However, normal cells can also produce tumor markers and elevated levels have been recorded even in non-cancerous conditions. This is why tumor marker tests have limited use in the diagnosis of cancer.
The perfect method of using tumor markers in cancer diagnosis is yet to be determined. Moreover, there have been controversies associated with the use of certain types of tumor marker tests. Currently, a variety of tumor markers are available such as calcitonin used for medullary thyroid cancer, prostate-specific antigen (PSA) used for prostate cancer, alpha-fetoprotein (AFP) used for liver cancer, human chorionic gonadotropin (HCG) used for germ cell tumors (ovarian and testicular cancer) and cancer antigen 125 (CA 125) used for ovarian cancer.
Hollevoet et al found that megakaryocyte potentiating factor (MPF) can be used as a serum biomarker of malignant mesothelioma. MPF originates from the same precursor protein as soluble mesothelin (SM), which is currently the reference serum biomarker for malignant mesothelioma. At 95% specificity, SM had a sensitivity of 64% (cutoff = 2.00nmol/L) and MPF had a sensitivity of 68% (cutoff = 12.38ng/mL). Combining both markers did not improve the diagnostic performance.
Pleural fluid findings in patients with mesothelioma are normally not diagnostic. The specific gravity of the pleural fluid is also nondiagnostic. Typically, the pleural fluid has less than 1000 leukocytes per microliter, few erythrocytes, elevated protein levels, and normal lactate dehydrogenase levels.
The results of cytologic examination are occasionally positive for malignant mesothelial cells; most often, however, the pleural fluid cytology results are not diagnostic.
Savic et al used fluorescence in situ hybridization (FISH) to distinguish malignant mesothelioma from reactive mesothelial cells in effusions. Diagnosis of mesothelioma by detection of chromosomal aberrations with FISH had 79% sensitivity; positive and negative predictive values for detection of mesothelioma were 100% and 72%, respectively.
Mesothelioma screening
Malignant mesothelioma is a rare and aggressive malignancy of the pleura (but also of other serous membranes), with a strong causal link to asbestos exposure. The diagnosis of mesothelioma is not straightforward, and pleural fluid cytology is a reliable diagnostic tool only in very experienced health centers.
Therefore a majority of patients require highly invasive procedures (such as video-assisted thoracoscopy or open biopsy) in order to adequately establish the diagnosis. Serum and cytological markers that have recently emerged could help in non-invasive confirmation or exclusion of mesothelioma.
Current recommendations for screening of patients who have been exposed to significant amounts of asbestos is that screening is not advised, as no effective early intervention that alters the outcome. Still, the principal aim of developing markers is to pursue a non-invasive diagnosis of mesothelioma in order to prevent invasive tests on the already weakened patient.
Serum markers
Early reliable diagnosis of malignant mesothelioma is extremely difficult as time intervals from exposure in the disease can be long and variable. Currently, there are no blood-based markers for routine use in clinical practice to diagnose malignant mesothelioma; however, promising biomarkers emerged in the recent years that help in establishing the correct diagnosis by indicating the need for a biopsy of the pleura at an earlier stage.
Mesothelin was identified as a potential biomarker for malignant mesothelioma over 10 years ago. It is a membrane-bound glycoprotein expressed by mesothelial cells and over expressed in mesothelioma. Soluble mesothelin related proteins (SMRP) represent a split product of such bound mesothelin, which is abundant on serosal cells of the pleura and peritoneum and involved in intracellular contact.
An enzyme-linked immunosorbent assay (ELISA test) using monoclonal antibodies against SMRP epitopes has been developed and validated by the Food and Drug Administration (FDA) for the measurement of SMRP levels in the circulation. Positive test for mesothelin at a high specificity threshold is a strong impetus for further diagnostic steps, if renal failure is excluded. However, the poor sensitivity of mesothelin at diagnosis (only up to 50%) limits the value of this biomarker.
According to the recent data, osteopontin has a great potential as a marker for mesothelioma with a positive predictive power equivalent to CA-125 for ovarian cancer. It is a type of glycoprotein involved in communication between cells, including signaling pathways involved in the development of cancer. Studies have suggested a sensitivity and specificity for mesothelioma of 77% and 85% respectively, although increased levels do not exclude other possible malignancies.
Megakaryocyte Potentiating Factor (MPF) is a soluble protein that is also produced by proteolytic cleavage of the mesothelin precursor protein. Recent studies show that MPF was elevated in 91% of malignant mesothelioma patients compared with controls, with its levels returning to normality after surgery in patients with peritoneal mesothelioma. Hence this marker is potentially useful in the monitoring of treatment response in mesothelioma.
The ideal serum marker for mesothelioma should offer early diagnosis, differentiation of this malignant entity from benign pleural disease or other metastatic pleural malignancies, proper identification of all the subtypes and survival prediction. Accurate quantification and reporting of the added value of mesothelioma markers are necessary for their clinical implementation.
Emerging prognostic markers
Resistance proteins involved in DNA repair mechanisms such as the excision repair cross-complementation group 1, mutL homolog 1 and βIII-tubulin are found to be associated with outcomes to specific, platinum-based type chemotherapy in patients with mesothelioma. Therefore the future use of these enzymes is to tailor platinum-based chemotherapy for malignant mesothelioma patients who may expect the largest clinical benefit.
CD24 immunoreactivity was recently recognized as potential novel marker in differentiating malignant mesothelioma from pulmonary adenocarcinoma. A short non-coding RNA molecule miR-126 in association with SMRPs acts as a marker for early detection of malignant pleural mesothelioma. Furthermore, another study suggested another micro-RNA (miR-625-3p) as a promising new candidate marker.
Recent studies pointed to secreted glycoprotein fibulin-3 as a marker with impressive diagnostic accuracy for the diagnosis of malignant mesothelioma, but also for differentiating subtypes of this disease. Effusion levels of fibulin-3 were significantly prognostic in assessing survival rates, thus further investigation of fibulin-3 is warranted since complete understanding of its biological role may result in new treatment options for malignant mesothelioma.
Characteristics of new cell lines
Four new mesothelioma cell lines have been characterized based on ultrastructural and immunophenotypic analysis. These cell lines express vimentin, cytokeratins 8 and 18, and mesothelial antigen recognized by HBME-1 monoclonal antibody. All of the lines possess surface human leukocyte antigen (HLA) class I and intercellular adhesion molecule-1 (ICAM-1).
Although HLA class II and cluster of differentiation-86 (CD-86) cannot be detected in the cell lines, HLA class II does become present following interferon gamma stimulation. Abnormal karyotypes with chromosome-6 abnormalities are found in all 4 cell lines.
Owing to the persistence of large T antigen with HLA class I and ICAM-1, large T antigen may serve as a target for cytotoxic-based immunotherapy.
Immunochemistry
Immunohistochemical studies play an important role for the pathologist in differentiating malignant mesothelioma from neoplastic mimics, such as breast or lung cancer that has metastasized to the pleura. There are numerous tests and panels available, but no single test is perfect for distinguishing mesothelioma from carcinoma or even benign versus malignant. The positive markers indicate that mesothelioma is present; if other markers are positive it may indicate another type of cancer, such as breast or lung adenocarcinoma. Calretinin is a particularly important marker in distinguishing mesothelioma from metastatic breast or lung cancer.
Immunohistochemistry results
Positive
|
Negative
|
EMA
(epithelial membrane antigen) in a membranous distribution
|
CEA (carcinoembryonic antigen)
|
WT1
(Wilms' tumour 1)
|
B72.3 (epithelial glycoprotein)
|
Calretinin
|
MOC-3 1 (tumor glycoprotein) / membranous
|
Mesothelin
|
CD15 (LeuM1) / membranous
|
Cytokeratin
5
|
Ber-EP4 (tumor glycoprotein) / membranous
|
HBME-1
(human mesothelial cell 1)
|
TTF-1 (thyroid transcription factor-1)
|
Podoplanin
(PDPN)
|
Claudin-4
|
Osteopontin
|
Epithelial cell adhesion molecule (EpCAM)
|
Estrogen receptor alpha
|
|
Mammaglobin
|
Histological findings
Histological Subtypes
There are three main histological subtypes of malignant mesothelioma: epithelioid, sarcomatous, and biphasic. Epithelioid and biphasic mesothelioma make up approximately 75-95% of mesotheliomas and have been well characterized histologically, whereas sarcomatous mesothelioma has not been studied extensively. Most mesotheliomas express high levels of cytokeratin 5 regardless of subtype.
- Epithelioid mesothelioma is characterized by high levels of calretinin.
- Sarcomatous mesothelioma does not express high levels of calretinin.
Gross pathology reveals that the pleural surfaces are seeded with malignant mesothelioma cells, which form grouped nodules. As the disease progresses, it covers the entire pleural space and invades the chest wall, mediastinum, and diaphragm. Microscopically, the 3 histologic types are epithelial, sarcomatous, and mixed. The epithelial type correlates with a better prognosis.
Other morphological subtypes have been described:
- Desmoplastic
- Clear cell
- Deciduoid
- Adenomatoid
- Glandular
- Mucohyaline
- Cartilaginous and osseous metaplasia
- Lymphohistiocytic
Cardiopulmonary stress test
A cardiopulmonary stress test with pharmacologic agents is a reasonable choice to eliminate the possibility of evidence of silent myocardial ischemia.
Laparoscopy, thoracoscopy, and pleuroscopy
Thoracoscopy or pleuroscopy should be performed to confirm the diagnosis of mesothelioma. Laparoscopy is important for staging but is still investigational with regard to its use in evaluation for transdiaphragmatic involvement.
Differential diagnosis of mesothelioma:
- Metastatic adenocarcinoma
- Pleural sarcoma
- Synovial sarcoma
- Thymoma
- Metastatic clear cell renal cell carcinoma
- Metastatic osteosarcoma
Other cancers related to asbestos exposure
Laryngeal Cancer
Although older studies conflict, a 2006 report sponsored by the National Institutes of Health indicated sufficient scientific evidence linking asbestos exposure to the development of laryngeal cancer. Cancer of the larynx, known as the voice box, is rare. The American Cancer Society estimates more than 12,000 cases will be diagnosed in 2012, the majority of which will be caused by smoking and heavy alcohol consumption.
Comparing the results of more than 50 epidemiological studies, the Institute of Medicine found that asbestos exposure significantly increases incidences of laryngeal cancer. There is also evidence that the risk increases with the intensity and duration of exposure. In addition, the study found that smoking, either alone or in combination with drinking, may contribute to the accumulation of asbestos fibers in the larynx.
Because the larynx lies directly in the path of an inhaled air stream, asbestos fibers can easily become lodged in the laryngeal mucosa. Other studies have suggested that asbestos-containing sputum can land on the larynx after being coughed up from the lungs.
Facts about laryngeal cancer :
- Incidence rates are more than four times higher in men
- Risk increases with tobacco use or excessive alcohol use
- Symptoms include abnormal breathing, cough and neck pain
Gastrointestinal Cancer
The World Health Organization already associates gastrointestinal cancers with asbestos exposure, and numerous studies have reported increased incidence of the cancers in exposed populations. Gastrointestinal cancers can involve tumors in a number of locations along the gastrointestinal tract. According to one major study of asbestos installation workers, the fibers are more likely to get trapped in the upper gastrointestinal tract (esophagus and stomach) than the lower sites (colon and the rectum).
Sites Affected:
- Esophagus
- Stomach
- Small Intestine
- Colon
- Rectum
- Gallbladder
- Pancreas
Asbestos-related gastrointestinal cancers are predominantly caused by chronic oral exposure to the mineral rather than short-term inhalation. Ingesting contaminated drinking water is thought to be one of the primary routes for fibers to reach gastrointestinal sites. Studies show fewer positive associations between asbestos and gastrointestinal cancers in regions of Utah and Connecticut, where community drinking water sources contained much lower concentrations of the mineral.
Facts about gastrointestinal cancer :
- Average age at the time of diagnosis is 61.4 years old
- Can arise in either the midgut, foregut or hindgut
- One study found 15,524 cases of gastrointestinal cancer among 12 different occupations known for asbestos exposure and mesothelioma
Colorectal Cancer
In 1986, the Occupational Safety and Health Administration added colorectal cancer to the list of illnesses to be screened for during asbestos surveillance examinations. While there is still no conclusive ruling on the link between colorectal cancer and exposure, many studies suggest that the two may be related. Fibers have been found in the colonic tissue of asbestos workers with colon cancer.
In one British study of asbestos cement workers, colorectal cancer had a clear relation to the cumulative dose of exposure. The duration of exposure was not found to impact the risk, but as the cumulative amount increased through the years, so did the risk. Colorectal cancer risk was found similar to the risk for respiratory cancers.
Facts about colorectal cancer :
- Colon and rectal cancers caused 51,690 deaths in 2012
- Surgery can cure approximately half of all patients
- A study found asbestos-related cases developed more often in the right part of the colon
Ovarian Cancer
A study by the International Agency for Research on Cancer (IARC) confirmed a causal relationship between asbestos exposure and ovarian cancer. Despite few documented cases of women exposed to asbestos and the misclassification of peritoneal mesothelioma as ovarian cancer on many death certificates, researchers found that exposure notably increases the likelihood of developing ovarian malignancies.
Occupational studies show excess mortality from reproductive cancers, yet there are inconsistencies between levels of exposure and incidences of ovarian cancer. Research showed that the toxic fibers can accumulate in the ovaries of exposed women, but the process of how they get there is under debate. Researchers suspect the use of talc on the genitals might be to blame. (Asbestos was a known ingredient in talc.) Evidence also showed that a father or husband who works with the mineral is a common denominator in many cases of this disease.
- Fifth most common cancer among women
- Causes more deaths than other female reproductive cancers
- Asbestos-contaminated talc has caused some cases
Gallbladder Cancer
The National Cancer Institute has determined that asbestos exposure can lead to an elevated risk of gallbladder cancer, but evidence for the correlation is still inconclusive. A positive correlation between asbestos and the disease in white females was found in two of seven studies, yet few others have been able to support the association.
- Incidence higher in ethnic populations.
- More common in women between the ages of 50 and 60
- A study found a connection between asbestos-tainted drinking water and female gallbladder cancers
Kidney Cancer
The first correlation between excess mortality from kidney cancer and asbestos was made in 1979. Asbestos has been found in human kidneys and in urine, leading to the belief that the fibers can cause cancerous changes in this organ. However, since the occurrence is rare, only a few studies have been able to explore the correlation.
Two out of three occupational studies revealed strong, direct evidence for an excess of kidney cancer mortality in exposed workers. A separate study found that kidney cancer was one of two cancers found more frequently among Utah residents whose water supply was funneled through asbestos-sided pipes. Despite these studies, others have provided inconclusive data to draw an official correlation.
Facts about Kidney cancer :
- Most common in men between the ages of 50 and 70
- Drinking asbestos-tainted water may affect cancer risk
- Renal cell carcinoma accounts for 90 percent of cases
ASBESTOSIS TREATMENT
There is no definitive treatment to reverse the effects of asbestos on the alveoli. Treatment focuses on slowing the progression of the disease and relieving symptoms. A patient will need routine follow-up care, such as chest X-rays and lung function tests, at regular intervals depending on the severity of the condition. There's no cure for asbestosis once it has developed, as it's not possible to reverse the damage to the lungs. Due to the accumulating damage caused by asbestos fibers, asbestosis and asbestos pleural disease are slowly progressive asbestos diseases. They may be treated, but not cured. The physician will try to ease the patient's symptoms and to prevent further medical complications.
` Physicians should remain aware of the complications of asbestosis in order to expedite detection and treatment. Inform patients about the work-related causation of the disease (potentially compensable) and report it to appropriate state or federal agencies. Additionally, advise smokers to quit smoking, and provide referral to a smoking cessation clinic.
There are no medications to cure this condition. Drugs are not effective in the treatment of asbestosis. Corticosteroids and immunosuppressive drugs do not alter the course of the disease. The control of asbestos in the workplace is the most effective method for preventing asbestosis. Cessation of further exposure to asbestos once the diagnosis of asbestosis is made is imperative because additional exposure increases the rate of progression. However, the disease may progress even after exposure has stopped.
Assessment of disease severity and functional impairment are important in tailoring a treatment and follow-up plan (ie, frequency of clinic visits, chest radiographs, pulmonary function testing).
The treatment of asbestosis requires prompt antimicrobial therapy for respiratory infections, as well as immunization against influenza and pneumococcal pneumonia. Assess the patient’s oxygenation status at rest and with exercise. If hypoxemia at rest or with exercise is detected, prescribe supplemental oxygen.
If the patient has been diagnosed with asbestosis, they will be advised to avoid further contact with asbestos and to quit smoking. Patients run an increased risk of respiratory infections, so the patient may be treated with antibiotics for other respiratory ailments. It is wise to avoid large crowds where they may be exposed to such ailments, and to keep influenza and pneumococcal immunizations up to date.
The patient can be instructed on how to perform bronchial drainage for the asbestosis. At home, the patient may also use an ultrasonic, mist humidifier to loosen bronchial secretions so that they can be expelled through coughing. Respiratory therapists can use chest physical therapy techniques to further aid in removing secretions. Shortness of breath is treated with bronchodilators, inhaled or oral medications that open up the bronchial tubes and allow the passage of air. In more severe asbestosis cases, supplemental oxygen may be required.
Productive cough is treated with humidifiers and chest percussion. For minor discomfort, the patient can take over-the-counter drugs such as acetaminophen or ibuprofen to reduce chest pain.
Unfortunately, patients with asbestosis and asbestos pleural disease have an increased chance of developing mesothelioma, asbestos lung cancer, and a variety of malignancies. The patient should be monitored for these asbestos diseases. Provide palliative care for the relief of distressing symptoms in advanced disease; provide hospice referral (preferably at home) when disease reaches the terminal phase.
There are some treatments that can be alleviating to the patient, such as:
- oxygen therapy – breathing in oxygen-rich air from a machine or tank to help improve breathlessness if the blood oxygen levels are low, delivered through the nostrils or by mask
- stop smoking if they smoke – symptoms can be worse in those who smoke, and smoking increases the risk of lung cancer. In smokers, medications may be prescribed to help quit, or to ease symptoms that may be related to cigarette-related lung problems.
- consider giving the flu vaccination and the pneumococcal vaccination – the lungs will be more vulnerable to infections like flu and pneumonia. Flu and pneumonia vaccines do not treat asbestosis, but are recommended for almost everyone with lung disease.
- Pulmonary rehabilitation, which is an exercise program sessions, designed to help all patients with chronic lung conditions, with discussion and advice to help manage the symptoms
Oxygen therapy at home is often necessary to relieve the shortness of breath and correct underlying low blood oxygen levels. Supportive treatment of symptoms includes respiratory physiotherapy to remove secretions from the lungs by postural drainage, chest percussion, and vibration. Nebulized medications may be prescribed in order to loosen secretions or treat underlying chronic obstructive pulmonary disease. Immunization against pneumococcal pneumonia and annual influenza vaccination is administered due to increased sensitivity to the diseases. Those with asbestosis are at increased risk for certain cancers. If the person smokes, quitting the habit reduces further damage. Periodic pulmonary function tests, chest x-rays, and clinical evaluations, including cancer screening/evaluations, are given to detect additional hazards. If the symptoms are very severe, the patient might be a candidate for a lung transplant consulted with a specialist. Consult a pulmonologist to assess the need for long-term oxygen therapy and for the management of advanced cases and complications. Because of the likelihood of bronchogenic carcinoma, consult a thoracic surgeon if a solitary pulmonary nodule develops in a patient with asbestosis.
MESOTHELIOMA TREATMENT
Conventional mesothelioma treatment specifically pleural mesothelioma can involve surgery, chemotherapy, radiation or a combination of two or more of these, which is known as multimodality therapy. These treatment methods can be curative, reducing the cancer and extending life expectancy, or they can be palliative, which means they are performed to alleviate cancer-related pain. Currently, no therapy is considered standard. The standard methods of surgery, radiation, or chemotherapy alone have not improved survival. In cases where standard treatments do not work, mesothelioma patients may also be able to try experimental treatments through clinical trials.
While no cure currently exists, mesothelioma patients can usually improve their prognosis through some form of treatment. Even in cases where improving lifespan is not viable, palliative care and alternative therapies often help reduce pain and suffering from symptoms for many individuals with mesothelioma.
Important considerations in determining a mesothelioma treatment plan include the cancer stage, primary site affected and cell type. Treatment options also depend on whether the cancer is localized to the chest or has spread to the chest wall, diaphragm, or lymph nodes as well as your age and overall health. The three standard therapies used to treat mesothelioma include surgery, chemotherapy, and radiation.
In addition to these traditional methods of cancer treatment, researchers are developing emerging techniques to fight the cancer. Cancer centers specializing in PM host clinical trials to test new drugs, treatment methods and other medical advancements.
Mesothelioma is generally resistant to radiation and chemotherapy treatment. Long-term survival and cures are exceedingly rare. Treatment of malignant mesothelioma at earlier stages has a better prognosis. Surgery in patients with disease confined to the pleural space is reasonable. Clinical behavior of the malignancy is affected by several factors including the continuous mesothelial surface of the pleural cavity which favors local metastasis via exfoliated cells, invasion to underlying tissue and other organs within the pleural cavity, and the extremely long latency period between asbestos exposure and development of the disease. The histological subtype and the patient's age and health status also help predict prognosis. The epithelioid histology responds better to treatment and has a survival advantage over sarcomatoid histology.
All types of mesothelioma are usually treated using a combination of three types of therapy:
- Surgery – Cytoreduction surgery (also called “debulking”) is often performed with the intent of removing as many cancer cells as possible.
- Chemotherapy – A combination of chemotherapy drugs (usually pemetrexed [Alimta] and Cisplatin) are administered to kill remaining tumor cells.
- Radiation – A blast of targeted radiation to shrink tumors in the body.
A. Surgery
Surgery, by itself, has proved disappointing. In one large series, the median survival with surgery (including extrapleural pneumonectomy) was only 11.7 months. However, research indicates varied success when used in combination with radiation and chemotherapy (Duke, 2008), or with one of the latter. A pleurectomy/decortication is the most common surgery, in which the lining of the chest is removed. Less common is an extrapleural pneumonectomy (EPP), in which the lung, lining of the inside of the chest, the hemi-diaphragm and the pericardium are removed. In localized pericardial mesothelioma, pericardectomy can be curative; when the tumor has metastasized, pericardectomy is a palliative care option. The entire tumor is not often able to be removed. For patients with an early-stage mesothelioma diagnosis, surgery can be used to remove all or most of the tumor(s). Depending on the tumor location, surgery may include removing the mesothelial lining, one or more lymph nodes, or part or all of a lung or other organ. Extrapleural pneumonectomy (EPP) and pleurectomy/decortication (P/D) are two surgeries that can potentially eliminate the cancer. The EPP removes the affected lung, parts of the chest lining, heart lining, nearby lymph nodes and part of the diaphragm. The P/D spares the affected lung but takes out the lining around it and tumors inside the chest cavity.
Younger, healthier patients fare best with surgery, but it's not effective for people with late-stage cancer or multiple tumors.
Pleurectomy and Pneumonectomy
Pleurectomy/Decortication (P/D)
This type of surgery involves removing the parietal pleura (the outer membrane) as well as a portion of nearby organs and tissue, such as the mediastinum, diaphragm, and pericardium. Although it can help treat many symptoms of mesothelioma – such as pleural effusion – it is not considered a curative operation, and results in recurrence about 80 – 90 percent of the time.
Because P/D is usually less stressful on the body, it is usually offered as a palliative treatment patients who have a later stage of the disease, or when curative options are not viable.
Measuring the diffusion capacity of the lung preoperatively is important because most patients have poor pulmonary reserve secondary to interstitial lung disease.
Surgical resection has been relied upon because radiation and chemotherapy have been ineffective primary treatments. The 2 surgical procedures used are pleurectomy with decortication and extrapleural pneumonectomy (EPP). A meta-analysis showed no statistically significant difference in 2-year mortality after pleurectomy with decortication compared with EPP, but pleurectomy with decortication was associated with a significantly lower proportion of short-term deaths (perioperatively and within 30 days) than EPP (1.7% vs 4.5%).
Pleurectomy with decortication is a more limited procedure and requires less cardiorespiratory reserve. It involves dissection of the parietal pleura, incision of the parietal pleura, and decortication of the visceral pleura, followed by reconstruction. It has a morbidity rate of 25% and a mortality rate of 2%. It is a difficult procedure because the tumor encases the whole pleura, and the local recurrence rate is high.
Extrapleural Pneumonectomy (EPP)
Patients who are in better physical condition can undergo EPP, which is a potentially curative treatment that removes the affected pleura along with a portion of the lung, nearby lymph nodes, and adjacent tissue. It is the only type of surgery associated with long-term survival.
Extrapleural pneumonectomy (EPP) is a more extensive procedure and has a higher mortality rate than P/D, although the mortality rate has improved, falling to 3.8%. The procedure involves dissection of the parietal pleura, division of the pulmonary vessels, and en bloc resection of the lung, pleura, pericardium, and diaphragm, followed by reconstruction. It provides the best local control because it removes the entire pleural sac along with the lung parenchyma.
With surgery alone, the recurrence rate is very high and most patients die after a few months. At least half of the patients who have local control with surgery have distant metastasis upon autopsy.
A study by Cao et al found that patients with nonepithelial malignant pleural mesothelioma and nodal involvement have a worse prognosis after extrapleural pneumonectomy, questioning their eligibility as candidates.
In patients with epithelioid-type malignant pleural mesothelioma who are fit enough to tolerate a thoracotomy, the best option is still a thoracotomy and macroscopic clearance of the tumor as part of multimodality therapy.
Because it is heavily invasive and stressful on the body, EPP is used almost exclusively for patients who meet the following criteria:
- Stage 1 or 2 mesothelioma
- Epithelial cell type mesothelioma
- Have not previously had coronary bypass surgery or pleurectomy
- Cardiac ejection fraction measurement of 45 percent or greater (this measures how well the heart pumps blood)
- No cardiac dysfunction and/or arrhythmia
- No liver, renal, or similar diseases
B. Radiation
Radiation therapy is commonly administered alongside chemotherapy and following surgery to kill any cancer cells the surgeon accidentally left behind. Radiation is most effective when used with other types of treatment, though it can provide some pain relief on its own. Through the use of targeted radiation, mesothelioma tumors can often be shrunk, making them easier to be removed through surgery. Depending on the tumor location, the radiation can be delivered using an external or an internal source.
In many cases, mesothelioma specialists will recommend a multimodal approach, which uses a combination of these three types of treatment. In various studies, multimodal treatment has been shown to be more effective than any of these individual treatments alone. For example, surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has resulted in an increase in the life expectancy of peritoneal mesothelioma patients in recent years.
For patients with localized disease, and who can tolerate a radical surgery, radiation can be given post-operatively as a consolidative treatment. The entire hemithorax is treated with radiation therapy, often given simultaneously with chemotherapy. Delivering radiation and chemotherapy after a radical surgery has led to extended life expectancy in selected patient populations. It can also induce severe side-effects, including fatal pneumonitis. As part of a curative approach to mesothelioma, radiotherapy is commonly applied to the sites of chest drain insertion, in order to prevent growth of the tumor along the track in the chest wall.
The results obtained with radiation therapy alone have been disappointing. Radiation has had no effect on survival, but it has provided significant palliation in 50% of patients treated for chest pain and chest wall metastasis.
However, investigators evaluating the feasibility of the Surgery for Mesothelioma After Radiation Therapy (SMART) approach for resectable malignant pleural mesothelioma reported that preoperative radiation therapy may improve survival. In a study of 25 patients with resectable malignant pleural mesothelioma, a 1-week course of high-dose hemithoracic intensity-modulated radiation therapy (IMRT) before extrapleural pneumonectomy proved feasible and prolonged survival. Cumulative 3-year survival was 84% among patients with epithelial subtypes (more than double the rate seen without IMRT), but it was 13% among those with biphasic subtypes. No grade 3 or higher toxicities were associated with IMRT.
Although mesothelioma is generally resistant to curative treatment with radiotherapy alone, palliative treatment regimens are sometimes used to relieve symptoms arising from tumor growth, such as obstruction of a major blood vessel. Radiation therapy, when given alone with curative intent, has never been shown to improve survival from mesothelioma. The necessary radiation dose to treat mesothelioma that has not been surgically removed would be beyond human tolerance. Radiotherapy is of some use in pericardial mesothelioma.
C. Chemotherapy
Chemotherapy involves treatment with a drug designed to kill cancer cells. It is usually administered by IV. The physician will determine dosage and frequency based on the patient’s health, weight and cancer stage. Chemotherapy drugs work by attacking fast-growing cells, such as cancer cells. Often used in conjunction with surgery, chemotherapy can kill any remaining mesothelioma cells that the surgeon was unable to remove physically.
While the effects of chemotherapy are immediate, it has a poor success rate in general and causes discomfort during infusions, despite that, chemotherapy is the only treatment for mesothelioma that has been proven to improve survival in randomised and controlled trials. Various doses / dosage are implemented to each specific different condition of the patient. The landmark study published in 2003 by Vogelzang and colleagues compared cisplatin chemotherapy alone with a combination of cisplatin and pemetrexed (brand name Alimta) chemotherapy in patients who had not received chemotherapy for malignant pleural mesothelioma previously and were not candidates for more aggressive "curative" surgery. This trial was the first to report a survival advantage from chemotherapy in malignant pleural mesothelioma, showing a statistically significant improvement in median survival from 10 months in the patients treated with cisplatin alone to 13.3 months in the group of patients treated with cisplatin in the combination with pemetrexed and who also received supplementation with folate and vitamin B12. Vitamin supplementation was given to most patients in the trial and pemetrexed related side effects were significantly less in patients receiving pemetrexed when they also received daily oral folate 500mcg and intramuscular vitamin B12 1000mcg every 9 weeks compared with patients receiving pemetrexed without vitamin supplementation. The objective response rate increased from 20% in the cisplatin group to 46% in the combination pemetrexed group. Some side effects such as nausea and vomiting, stomatitis, and diarrhoea were more common in the combination pemetrexed group but only affected a minority of patients and overall the combination of pemetrexed and cisplatin was well tolerated when patients received vitamin supplementation; both quality of life and lung function tests improved in the combination pemetrexed group. In February 2004, the United States Food and Drug Administration approved pemetrexed for treatment of malignant pleural mesothelioma. However, there are still unanswered questions about the optimal use of chemotherapy, including when to start treatment, and the optimal number of cycles to give. Cisplatin and pemetrexed together give patients a median survival of 12.1 months.
Cisplatin in combination with raltitrexed has shown an improvement in survival similar to that reported for pemetrexed in combination with cisplatin, but raltitrexed is no longer commercially available for this indication. For patients unable to tolerate pemetrexed, cisplatin in combination with gemcitabine or vinorelbine is an alternative, or vinorelbine on its own, although a survival benefit has not been shown for these drugs. For patients in whom cisplatin cannot be used, carboplatin can be substituted but non-randomised data have shown lower response rates and high rates of haematological toxicity for carboplatin-based combinations, albeit with similar survival figures to patients receiving cisplatin. Currently, cisplatin as a single drug has been used as the standard drug for phase III clinical trials. None of the standard treatment options has improved survival. The most active agents are anthracycline, platinum, and alkylating agents; each produces a response rate of 10-20%.
In January 2009, the United States FDA approved using conventional therapies such as surgery in combination with radiation and or chemotherapy on stage I or II Mesothelioma after research conducted by a nationwide study by Duke University concluded an almost 50 point increase in remission rates.
In pericardial mesothelioma, chemotherapy - typically adriamycin and/or cisplatin - is primarily used to shrink the tumor and is not curative.
Pemetrexed disodium was approved by the US Food and Drug Administration (FDA) to treat patients with malignant pleural mesothelioma in unresectable disease and those who are not candidates for curative surgery. Several trials from a combination drug to therapy with pemetrexed have been performed. Hughes et al showed a 32% response rate using pemetrexed 500 mg/m2 and carboplatin (area under the curve [AUC] of 5) on an every-21-day schedule. An interesting combination of drugs, including raltitrexed and oxaliplatin, has shown a response rate of 20% in previously treated patients.
C.1. Chemotherapy drugs summary
a. Antineoplastic Agents
These agents interfere with cell reproduction. Some agents are cell-cycle specific, while others (eg, alkylating agents, anthracyclines, cisplatin) are not phase specific. Cellular apoptosis is also a potential mechanism of many antineoplastic agents.
Gemcitabine (Gemzar)
Gemcitabine is a cytidine analogue that, after being metabolized intracellularly to an active nucleotide, inhibits ribonucleotide reductase and competes with deoxycytidine triphosphate for incorporation into deoxyribonucleic acid (DNA). It is cell-cycle specific for the S phase.
Pemetrexed disodium (Alimta)
This agent disrupts folate-dependent metabolic processes essential for cell replication. It specifically inhibits thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT), which are folate-dependent enzymes involved in de novo biosynthesis of thymidine and purine nucleotides. Pemetrexed disodium is indicated for use in combination with cisplatin to treat patients with malignant pleural mesothelioma in unresectable disease, as well as patients who are not candidates for curative surgery.
Cisplatin (Platinol)
Cisplatin is a platinum-based alkylating agent. It inhibits DNA synthesis and, thus, cell proliferation by causing DNA crosslinks and denaturation of the double helix. Cisplatin is indicated for use in combination with pemetrexed disodium to treat patients with malignant pleural mesothelioma in unresectable disease and those who are not candidates for curative surgery.
Doxorubicin (Adriamycin)
Doxorubicin is an anthracycline antibiotic that causes DNA strand breakage through effects on topoisomerase II and direct intercalation into DNA, which causes DNA polymerase inhibition. This drug is both mutagenic and carcinogenic.
b. Antineoplastics, Monoclonal Antibody
Bevacizumab (Avastin)
Recombinant humanized monoclonal antibody to VEGF; blocks the angiogenic molecule VEGF thereby inhibiting tumor angiogenesis, starving tumor of blood and nutrients. Bevacizumab has orphan drug designation for treatment of mesothelioma
C.2. Chemotherapy drug combinations
Cisplatin/pemetrexed
In a phase III study, Vogelzang et al showed the superior benefits of a regimen using pemetrexed in combination with cisplatin over administration of cisplatin alone. Pemetrexed (500 mg/m2/day) and cisplatin (75 mg/m2/day) or cisplatin alone (75 mg/m2/day) was given on day 1. Both arms were given every 21 days. The median survival time in the cisplatin/pemetrexed arm was 12.1 months versus 9.3 months for cisplatin alone. The response rate was 41.3% for the cisplatin/pemetrexed arm and 16.7% for the cisplatin arm. Folic acid and vitamin B-12 were given routinely to prevent the adverse effects of pemetrexed. This trial established the regimen as the standard of care for this disease.
Santoro et al reported that chemonaive patients with malignant pleural mesothelioma who received either pemetrexed/cisplatin or pemetrexed/carboplatin had similar time to progressive disease and 1-year survival rates. The response rate in the pemetrexed/cisplatin group was 26.3%, compared with 21.7% for the pemetrexed/carboplatin group. The 1-year survival rates were 63.1% and 64%, respectively, and the median times to progressive disease were 7 and 6.9 months, respectively.
Cisplatin/pemetrexed/bevacizumab
In the open-label phase III Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS), Zalcman et al reported significantly longer overall survival with the addition of bevacizumab to the cisplatin/pemetrexed regimen as first-line treatment of advanced malignant pleural mesothelioma. Median survival in the 223 patients treated with cisplatin/pemetrexed/bevacizumab was 18.8 months (95% confidence index [CI] 15.9–22.6]), versus 16.1 months (95% CI, 14.0–17.9; hazard ratio 0.77 [0.62–0.95]; P=0.0167) in the 225 patients treated with cisplatin/pemetrexed. However, patients receiving bevacizumab experienced more grade 3 or higher hypertension (23% vs 0%) and thrombotic events (6% vs 1%).
Pemetrexed/gemcitabine
As first-line chemotherapy for patients with peritoneal mesothelioma, the combination of pemetrexed plus gemcitabine is active and can be an option for patients who cannot take cisplatin. A phase II study of gemcitabine 1000 mg/m2 on day 1 and day 8 and pemetrexed 500 mg/m2 day 8 every 21 days for 6 cycles or until progression showed a response rate of 15% (95% CI, 3.2-37.9%), with 3 patients exhibiting partial response. The disease control rate was 50%. The most common nonhematologic toxicities included fatigue (20%), constipation (10%), vomiting (10%), and dehydration 10%. Hematologic toxicities included neutropenia (60%) and febrile neutropenia (10%).
Single-agent pemetrexed
Single-agent pemetrexed therapy showed a response rate of 10.5%, a median time to progressive disease of 6 months, and a median survival time of 14 months in chemo-naive patients. Of the pretreated patients, the response rate was 12.1% and median time to progressive disease was 4.9 months.
Cisplatin/gemcitabine
A 1999 phase II study by Byrne et al using cisplatin (100 mg/m2) on day 1 and gemcitabine (1000 mg/m2) administered intravenously on days 1, 8, and 15 of a 28-day cycle for 6 cycles showed response rates of 47.6% (complete and partial response), 42.8% (stable disease), and 9.5% (progressive disease). The median response duration was 25 weeks, progression-free survival was 25 weeks, and the overall survival was 41 weeks. Toxicity was mainly gastroenterologic and hematologic in nature.
Additional drug combinations
Several other combinations have been found to be active, including cisplatin / doxorubicin (Adriamycin) / mitomycin C, bleomycin / intrapleural hyaluronidase, cisplatin / doxorubicin (Adriamycin), carboplatin / gemcitabine, and cisplatin / vinblastine / mitomycin C. The cisplatin / gemcitabine combination has yielded the best results.
Additional chemotherapy research
With the isolation of mesothelial cell lines, several chemotherapeutic agents are being tested to assess their efficacy. One explanation for the poor response to chemotherapy is the low apoptotic rate, as evidenced by low BCL2 and BAX expression. These data suggest that apoptosis is not a key phenomenon in mesothelioma development and histologic differentiation.
Numerous trials of chemotherapeutic agents have been performed; until recently, however, the studies were small, the staging systems used were different, and the measurements of disease were inaccurate.
In July 2013, the cancer stem cell inhibitor defactinib (VS-6063) received an orphan drug designation from the US Food and Drug Administration (FDA) for treatment of mesothelioma. The drug's manufacturer agreed to conduct a double-blind, placebo-controlled trial in patients with malignant pleural mesothelioma. However, the trial was terminated when interim analysis showed a good safety profile but lack of efficacy.
Immunotherapy
Treatment regimens involving immunotherapy have yielded variable results. For example, intrapleural inoculation of Bacillus Calmette-Guérin (BCG) in an attempt to boost the immune response, was found to be of no benefit to the patient (while it may benefit patients with bladder cancer). Mesothelioma cells proved susceptible to in vitro lysis by LAK cells following activation by interleukin-2 (IL-2), but patients undergoing this particular therapy experienced major side effects. Indeed, this trial was suspended in view of the unacceptably high levels of IL-2 toxicity and the severity of side effects such as fever and cachexia. Nonetheless, other trials involving interferon alpha have proved more encouraging with 20% of patients experiencing a greater than 50% reduction in tumor mass combined with minimal side effects.
Heated intraoperative intraperitoneal chemotherapy
This technique is used in conjunction with surgery, including in patients with malignant pleural mesothelioma. The surgeon removes as much of the tumor as possible followed by the direct administration of a chemotherapy agent, heated to between 40 and 48 °C, in the abdomen. The fluid is perfused for 60 to 120 minutes and then drained. High concentrations of selected drugs are then administered into the abdominal and pelvic surfaces. Heating the chemotherapy treatment increases the penetration of the drugs into tissues. Also, heating itself damages the malignant cells more than the normal cells.
Multimodality therapy (combination of more than one method of treatment)
All of the standard approaches to treating solid tumors—radiation, chemotherapy, and surgery—have been investigated in patients with malignant pleural mesothelioma. Although surgery, by itself, is not very effective, surgery combined with adjuvant chemotherapy and radiation (trimodality therapy) has produced significant survival extension (3–14 years) among patients with favorable prognostic factors. However, other large series of examining multimodality treatment have only demonstrated modest improvement in survival (median survival 14.5 months and only 29.6% surviving 2 years). Reducing the bulk of the tumor with cytoreductive surgery is key to extending survival. Two surgeries have been developed: extrapleural pneumonectomy and pleurectomy/decortication. The indications for performing these operations are unique. The choice of operation namely depends on the size of the patient's tumor. This is an important consideration because tumor volume has been identified as a prognostic factor in mesothelioma. Pleurectomy/decortication spares the underlying lung and is performed in patients with early stage disease when the intention is to remove all gross visible tumor (macroscopic complete resection), not simply palliation. Extrapleural pneumonectomy is a more extensive operation that involves resection of the parietal and visceral pleurae, underlying lung, ipsilateral (same side) diaphragm, and ipsilateral pericardium. This operation is indicated for a subset of patients with more advanced tumors, who can tolerate a pneumonectomy.
Multimodal treatment typically consists of a primary treatment used in combination with a neoadjuvant therapy (a “helper” treatment before the primary treatment) or an adjuvant treatment (a helper treatment after the primary treatment). For example, one multimodal approach might include:
- Neoadjuvant therapy: Radiation to shrink the tumor size
- Primary treatment: Surgery to remove the tumor
- Adjuvant therapy: Chemotherapy to kill any remaining cells
The treatment plan should be developed in conjunction with a mesothelioma specialist who can provide details about the specific therapy approach being taken.
Trimodality therapy involves a combination of all 3 standard strategies: surgery, chemotherapy, and radiation. In a study, patients undergoing a trimodality approach involving extrapleural pneumonectomy followed by combination chemotherapy and radiotherapy had an overall median survival rate of 24% at 2 years. Seven patients were still alive at the end of the study, including 2 patients who by that time had survived for 40-45 months. Lymph node involvement was a significant negative prognostic factor in the study. The median length of survival for patients with lymph node metastasis was 13 months, while the median length of survival for patients without lymph node involvement was 24 months. Patients with the epithelial type of mesothelioma had a better survival rate than did patients with the sarcomatous or mixed type (65% vs 20% at 2y and 27% vs 0% at 5y, respectively).
Chemotherapeutic regimens found to be useful in the trimodality treatment include cyclophosphamide/doxorubicin (Adriamycin)/cisplatin, carboplatin/paclitaxel, and cisplatin/methotrexate/vinblastine. External beam radiotherapy is delivered in a standard fractionation over 5.5-6 weeks.
Experimental Alternative Treatments
Although emerging and experimental treatments can be risky because they have not yet proven effective, they can lead to improvements of traditional cancer therapies.
For example, in recent immunotherapy clinical trials, researchers boosted the immune systems of some pleural mesothelioma patients to significantly minimize cancer symptoms and tumor progression.
Alternative Therapies
Many patients with PM also use integrative oncology or complementary and alternative treatments, such as massage and yoga, to relieve pain and other side effects of treatment. While these therapies cannot cure cancer, they can improve your quality of life and relieve stress.
Alternative therapies include:
- Manipulative and body-based methods
- Energy therapies
- Exercise therapies
- Mind-body interventions
- Spiritual therapies
- Nutritional therapeutics
Diet and Activity
Patients are usually cachetic after surgery, chemotherapy, and radiation. Good supportive care and a regular nutritional status assessment are warranted. Patients should be referred to a nutritionist.
Beginning physical activity as soon as possible is important to prevent postoperative complications. Pulmonary physiotherapy is very helpful because of the extensive lung resection in patients with malignant pleural mesothelioma.
Follow-up
Regular follow-up visits with an internist, pulmonary specialist, medical oncologist, and radiation oncologist are recommended.
Consultations and Referrals
A good working relationship among the occupational medicine specialist, the environmental hazard team, and the community at large is important.
If an infection is suggested initially, consultation with a pulmonary specialist is essential if the infection does not resolve within 2 weeks with adequate antibiotic treatment. Chest radiographs are mandatory for follow-up if the infection has resolved. If the patient has diffuse calcification of the pleura and a history of weight loss with chronic cough, a full evaluation by a pulmonary specialist and oncologist is necessary.
A referral for thoracoscopy is warranted if the diagnosis is considered and the initial workup is not diagnostic.
Prognosis of mesothelioma
Survival based on the Brigham staging system for mesothelioma was as follows:
- Stage I: 22 months
- Stage II: 17 months
- Stage III : 11 months
Overall median survival was 17 months, yielding a 2-year survival rate of 36% and a 5-year survival rate of 14%. Survival in patients with epithelial cell mesothelioma was better, with a 2-year survival rate of 68% and 5-year survival rate of 46%.
LEGAL ASPECTS OF ASBESTOSIS AND MESOTHELIOMA
A. IN THE UNITED KINGDOM
If a person is diagnosed with an asbestos-related disease, he or she might get compensation or financial assistance. Depending on the circumstances, this can happen through the courts, the benefits system or government compensation schemes.
The person may wish to pursue a civil claim against previous employers, where exposure to asbestos may have occurred during that employment. People across the UK who have asbestos-related conditions can apply for industrial injuries benefits if they have one of the conditions known as a ‘prescribed disease’. These are:
- Asbestosis (pneumoconiosis)
- Diffuse mesothelioma
- Primary carcinoma (cancer) of the lung with asbestosis
- Primary carcinoma (cancer) of the lung without asbestosis but where there has been extensive occupational exposure to asbestos in specified occupations
- Unilateral or bilateral diffuse pleural thickening.
There are also government compensation schemes under the Pneumoconiosis etc (Worker’s Compensation) Act 1979 and the Diffuse Mesothelioma Payment Scheme.
Who is entitled for a compensation?
If a person has been diagnosed with asbestosis, he or she may be able to claim compensation through:
- Industrial injuries disablement benefit
- A claim for governmental compensation under the Pneumoconiosis etc. (Workers' Compensation) Act 1979
- A civil claim for compensation against previous employers
According to industrial injuries disablement benefit on the GOV.UK website. The aforementioned person can also get advice on benefits and compensation on the British Lung Foundation website.
Industrial Injuries Disablement Benefit (IIDB)
A person might get Industrial Injuries Disablement Benefit (IIDB) if that person became ill or are disabled because of an accident or disease either:
- At work
- The amount they may get depends on your individual circumstances.
- On an approved employment training scheme or course
What they will get
The level of the disability will affect the amount of benefit the person may get. This will be assessed by a ‘medical advisor’ on a scale of 1 to 100%.
Normally they must be assessed as 14% disabled or more to get the benefit.
All amounts are a guide only.
Assessed level of disablement
|
Weekly amount
|
100%
|
£169.70
|
90%
|
£152.73
|
80%
|
£135.76
|
70%
|
£118.79
|
60%
|
£101.82
|
50%
|
£84.85
|
40%
|
£67.88
|
30%
|
£50.91
|
20%
|
£33.94
|
Eligibility
The person may be able to claim Industrial Injuries Disablement Benefit (IIDB) if:
- He/she were employed when the accident or event happened
- He/she were on an approved employment training scheme or course when the accident or event happened
- The work accident or event that caused the illness or disability happened in England, Scotland or Wales
There are some exceptions according to different regions where you can ask Industrial Injuries Disablement Benefit Centre about.
Reforming the legal system
The legal process for mesothelioma claims can be complex, lengthy and expensive. The insurance industry is campaigning for improved processes that will allow mesothelioma sufferers to settle their cases more quickly.
Mesothelioma Support Scheme
In January 2014 the United Kingdom (UK) Government passed the Mesothelioma Act 2014 legislation to establish the Diffuse Mesothelioma Payment Scheme (DMPS). The scheme is funded by the insurance industry and will make payments to around 3,000 mesothelioma sufferers who cannot find an employer or insurer to claim from. Mesothelioma sufferers diagnosed after 25 July 2012 who are unable to trace an insurer or compensator are eligible to make a claim under the scheme. The first payments will be made in July 2014. A levy imposed on employers' liability insurers will fund the scheme at an estimated cost of £35 million a year. For more information see the DMPS website.
Tracing insurers and former employers
Mesothelioma sufferers may find it difficult to track down their former employer’s employers' liability insurer if the company has gone out of business. In April 2011 the insurance industry set up the Employers’ Liability Tracing Office (ELTO) to help people find insurers if their former employer is no longer in business. The ELTO has a database of millions of insurance policies from all past and present employers' liability insurers. The service has made tracing policies considerably easier for mesothelioma sufferers.
Over the next few month the ABI and the ELTO will be introducing further improvements. These will include a committee to analyse claimants' evidence of the existence of their former employer's employers' liability policy in the absence of the policy itself.
B. IN THE UNITED STATES
Who is eligible for Compensation?
Determining whether a person is eligible for compensation depends on the type of compensation they are seeking.
Settlement and Verdict Award Eligibility
While there is no specific set of criteria for seeking compensation through a mesothelioma settlement or jury award, there are a number of factors that will help you build a solid legal case:
- A confirmed mesothelioma diagnosis from a reputable doctor
- Complete and accurate records of all your medical costs related to diagnosis and treatment
- Occupational records that show you worked at a known job site containing asbestos
- The names and specific products or materials that you handled while employed there
- Similar details about other exposures that may have occurred at home, school, or elsewhere
Essentially, the more detailed information you have about your exposure to asbestos and the resulting disease, the better you can build a case for receiving compensation. Consulting with mesothelioma lawyers who understand the appropriate legal precedents and evidence requirements is the first step to determining your eligibility for mesothelioma compensation from a settlement or verdict.
Compensation Eligibility for Asbestos Trusts
Typically, to be eligible for compensation from an asbestos bankruptcy trust fund, the person will need to meet that fund’s specific eligibility requirements. These requirements are established in advance by the fund’s board of trustees, and they typically include a specific list of sites where asbestos exposure occurred. An experienced mesothelioma lawyer can evaluate eligibility for each asbestos trust fund and maximize compensation for mesothelioma victims from those funds.
As of 2016, there are currently about 60 asbestos trusts operating in the United States, each having their own eligibility requirements. Given the large number of trusts and the wide variation in their rules, the best way to seek compensation from an asbestos trust is to consult with a lawyer who has handled asbestos trust related compensation claims in the past and can explain the eligibility requirements for the patient.
Veteran Compensation Eligibility
According to the VA, veterans must meet three eligibility requirements to receive compensation for mesothelioma:
- Discharge under other than dishonorable conditions (i.e., honorable discharge, discharge under honorable conditions, and general discharge)
- Exposure to asbestos must have occurred during your military service
- The disease or disability must be related to the asbestos exposure that occurred while serving in the military
To prove eligibility, the VA requires evidence indicating that the exposure to asbestos happened during your time of military service, and that the exposure led to a disease or disability. As part of the evidence-gathering process, the VA will require a medical examination.
Note that simply being exposed without being diagnosed with mesothelioma or another asbestos-related disease is not enough to qualify for VA disability benefits.
How Much Compensation Can A Person Get?
There is no set amount of compensation for mesothelioma claims. Each case needs to be assessed individually, and the amount of money a person receive will depend on a wide variety of factors, including but not limited to:
- Where they have lived, worked, or served in the military
- Their age now, when they were exposed to asbestos, and when they received a mesothelioma diagnosis
- What products or materials containing asbestos they were exposed to
- How much physical or mental anguish (pain and suffering) they experienced as a result of the disease
In addition, the compensation amount will include consideration of how much money they have spent, as well as wages lost, due to the disease, including:
- Lost income from being out of work
- The inability to support dependents
- Medical bills for diagnosis and treatment
- Travel expenses to and from treatment centers
- Funeral costs
Jury awards potentially could also include punitive damages to penalize the company liable for the asbestos exposure.
Common expenses for compensation of mesothelioma patients:
- Lost wages
- Therapy and grief support
- Caregiver costs
- Travel expenses for treatment
- Funeral expenses
- Other expenses not covered by insurance
Mesothelioma Case Verdict Examples
Mesothelioma lawsuits have helped hundreds of thousands of people seek compensation from companies that negligently exposed them to asbestos. The ultimate goal is to put money in the hands of someone who needs help reducing financial hardships during an illness and providing a more stable future for loved ones. A few examples of legal option outcomes in the united states:
- 1.74 million dollars. In 2011, the Delaware Supreme Court awarded damages to the family of a mother and son who died of pleural mesothelioma after exposures at a family auto shop. Four surviving family members received $1.24 million for pain and suffering and $500,000 in a wrongful death verdict.
- 18.6 million dollars. A Dallas county jury awarded $18.6 million in 2014 to the family of a tire builder who died of mesothelioma. The Goodyear Tire & Rubber Co. employee was repeatedly exposed to asbestos over 30 years while working with Goodyear tire machines at a plant in Tyler, Texas.
- 115 million dollars. In 1998, a Texas jury awarded $115 million to 21 steelworkers who developed asbestosis while working at an Alabama steel mill. Carborundum Company, the manufacturer of an asbestos-containing grinding wheel used at the mill, was ordered to pay $100 million in punitive damages.
- 250 million dollars. A retired U.S. Steel worker from Indiana won a 2003 mesothelioma trial after alleging the company was responsible for exposing him to asbestos insulation for decades. U.S. Steel was expected to appeal the $250 million verdict, but instead settled out of court for an undisclosed amount.
Types of Asbestos Compensation
In general, there are four types of compensation available for mesothelioma claims.
1. Settlements
One way to receive compensation for asbestos-related diseases is through a legal settlement. To receive a settlement, it is first necessary to file a claim against one or more companies for either personal injury or wrongful death. Often times, companies will prefer to settle such claims rather than to risk a full trial, which could result in them having to pay even more money. Even if a case does go to trial, a claim may still be settled before the final verdict is issued.
The majority of mesothelioma lawsuits result in settlements. Having a team of knowledgeable lawyers who have demonstrated an ability to successfully navigate the settlement process is the most certain way to receive the maximum compensation for mesothelioma victims and their families.
2. Compensation for Veterans
Retired military personnel – especially those who served in the Navy, Coast Guard, Merchant Marine, and U.S. Army Transport Service – comprise one of the largest populations of people who were exposed to asbestos, due to the use of this material in warships and other naval vessels. As a result, the U.S. Department of Veterans Affairs (VA) has designated mesothelioma as one of several diseases that qualify for disability benefits.
The available benefits for veterans exposed to asbestos is dependent upon certain criteria—a veteran must have been discharged under circumstances other than dishonorable conditions and show that the asbestos exposure leading to mesothelioma occurred during the time of his/her service. Veterans may also be eligible to receive no-cost health benefits through the VA for service-related disabilities, including mesothelioma.
3. Jury Awards
From time to time, a mesothelioma claim may make it all the way to a jury trial. If no settlement is reached, and if the defendant is found liable for the asbestos exposure that led to the development of mesothelioma, a jury will determine the amount of compensation to be paid to the injured party. The amount awarded often includes actual expenses incurred as well as punitive damages.
Waiting for a verdict can sometimes be risky, which is why many companies want to settle. However, it may also be in the best interest of the plaintiff to settle as well, since juries could also return a verdict that either finds the company not to be liable or which awards a smaller amount of compensation than they might have received as a settlement. Having a skilled attorney who can advise you about the strength of your case and the likelihood of a favorable verdict and award is critical to ensuring that, as a mesothelioma victim, you receive the greatest remuneration possible.
4. Asbestos Bankruptcy Trust Funds
Exposure to asbestos has a long history, and over time a number of companies that manufactured, distributed, sold, or used asbestos products and materials have either gone out of business, merged with other corporations, or been restructured due to bankruptcy. In some cases, the downfall of these companies was due directly to the liability they incurred because of their use of asbestos.
Even though such companies no longer exist, it may still be possible to receive compensation from them. In many instances, the companies were required to establish asbestos bankruptcy trust funds (“asbestos trusts”) and fund them with enough money to pay mesothelioma claims brought against them. These mesothelioma trust funds typically have a set of established criteria, and mesothelioma victims who meet those criteria can expect to receive compensation from the fund.
Legal options
Asbestos exposure is often the result of someone else’s negligence, making mesothelioma an almost entirely preventable cancer. Many companies knew of the dangers of asbestos but failed to warn their employees. Lawsuits hold these companies accountable while providing much-needed compensation to those diagnosed with asbestos-related diseases.
A qualified mesothelioma lawyer will help you decide if it’s better to file a lawsuit or pursue another type of claim, as well as estimate the potential value of your claim.
Depending on your situation, it may even be possible for you to receive compensation without stepping foot in court. Many people have successfully done so through asbestos trust claims, VA disability claims and out-of-court settlements.
Filing a Lawsuit in Court
There are two types of asbestos injury lawsuits: Personal injury claims and wrongful death suits. Specific rights in these lawsuits depend on the county and state where the case is filed. A mesothelioma lawyer will help you determine which of these lawsuits is best for you or your family.
Personal Injury Claim: An individual diagnosed with mesothelioma can file a personal injury claim against one or more companies that may be responsible for their illness. Many cases are settled out of court.
Wrongful Death Suit: The family of a person who died from the asbestos-related disease can file a wrongful death lawsuit. There is a possibility they may need to appear in court.
Filing a Trust Claim Outside of Court
More than 60 trusts have been established on behalf of asbestos companies that filed for bankruptcy reorganization to avoid future lawsuits. The U.S. government requires these companies to fund these trusts with enough money to pay out current and future claims.
It is estimated that asbestos trust funds contain more than $30 billion in total.
Filing for VA Benefits
Military veterans can apply for VA benefits such as health care and monthly disability compensation. The surviving spouses and children of veterans who died of service-related disabilities can apply for a monthly Dependency and Indemnity Compensation (DIC) benefit.
According to the 2016 Veterans Compensation Benefits Rate Tables, an unmarried veteran without children can receive more than $2,900 per month, while a married veteran with at least one child can receive nearly $3,200 per month.
Lawsuit Settlements
You may file a lawsuit and win compensation without having to go to court. The vast majority of mesothelioma lawsuits never go to trial. Although a jury verdict may result in a larger reward, mesothelioma settlements are guaranteed compensation and a quicker resolution to a case.
Trials can be lengthy and some plaintiffs might not receive compensation until months after the verdict, depending on an appeal. An experienced lawyer will explain the strengths of your case and help you decide whether to settle or seek a verdict.
Attorney fee
In asbestos lawsuits, attorneys work on a contingency fee basis. This means you pay nothing until you receive compensation and nothing at all if your case is not successful. Contingency fees protect you because they usually are based on a percentage of any compensation you receive. Be sure to discuss this percentage during your first meeting with an lawyer.
Tips on hiring a lawyer/attorney to help resolve an asbestosis mesothelioma case:
- Check if the attorney has a proven track record winning asbestos lawsuits or large settlements.
- Find out if the attorney will travel to you when gathering information for the case.
- Ask about the attorney’s experience filing successful claims against asbestos trust funds.
- See if the attorney will evaluate your potential compensation.
INSURANCE
What Does Insurance Actually Cover?
Insurance coverage depends on each particular plan, but most of the diagnostic testing and treatments associated with mesothelioma should be covered in the plan. There are often high deductibles to reach and copays — both of which add to the cost of care — but having health insurance is considerably better than not having any coverage.
Cost estimates for care could vary, depending on many factors, but they typically are out of reach for most families without health insurance or other ways to pay.
An eight-week cycle of chemotherapy could cost $30,000. Monthly radiation treatments can be more than $2,000. An average surgery, which doesn’t include the largest, most aggressive ones, could cost $40,000. Having insurance will cut those costs significantly or eliminate them completely.
Private Insurance
Private health insurance options are either group health plans or individual health plans, but they work in a similar fashion.
Group plans cover many people, usually employees and dependents of those workers. The employers often pay a portion of the monthly premiums to provide their workers coverage.
The individual plans cover individual workers, often dependents, and are sold directly by an insurance company. Higher premiums generally mean more complete coverage.
Public Insurance
Medicare and Medicaid are the largest federally funded public health insurance programs in the U.S., although both are administered by the states which adjust them accordingly.
Medicare typically covers those over the age of 65 who have paid into the system throughout their working lives. There are four main parts in Medicare that will determine what is excluded or included in each person’s coverage:
- Part A: Everyone involved receives this. It covers inpatient care in hospitals, inpatient care in a nursing, hospice or home facility.
- Part B: Costs an additional premium each month and includes doctor visits, laboratory costs, medical equipment and ambulance care.
- Part C: It also costs an additional fee and is a combination of parts A and B. It’s provided by private insurance companies as supplemental insurance.
- Part D: Helps pay for prescription drugs.
Medicaid is designed to cover the cost of medical care for those below a certain income level who did not qualify for Medicare. Not all health care providers accept it, and the benefits can vary from state to state, but mesothelioma treatment can be obtained.
Limitations
Many health insurance plans have a broad network of providers that offer a variety of choices for your care. Others have narrower networks and choices are limited, which becomes a problem, but not a door closer.
Mesothelioma presents a unique set of health insurance problems because it is so rare. Few medical professionals are familiar with it and comfortable treating it effectively. To get the best care, patients should locate a mesothelioma specialist, even when it means going outside the network, and meeting a higher deductible, to do so.
Talk to your local oncologist or your primary care physician and explain your insistence to see a mesothelioma specialist. They can help facilitate the approval to go outside of network if necessary. Medicare and Medicaid patients who need to go outside their state will encounter additional hurdles, but they are not insurmountable.
What Happens When a Claim Is Denied?
If a claim is denied by your insurance company or you are told your coverage doesn’t include a particular mesothelioma specialist, don’t stop there. Don’t get discouraged. And don’t take no for an answer. Be your own advocate.
“A lot of times, people just give up when they are told no,” said Missy Edmunds, medical outreach director and patient advocate at Asbestos.com. “But you can’t give up. You re-submit the request. You go back to your doctor, and talk to him about it. We work with patients to help get them approved.”
Contact a patient advocate at Asbestos.com who can help you work through the process. They know the specialists who will help facilitate the process. It’s not easy to navigate through the health insurance maze, but it’s possible.
Affordable Care Act
The Affordable Care Act (ACA) went into effect in 2014 and made it easier for low-income families to acquire health insurance through government subsidies. It also set minimum health insurance standards that include cancer screenings, cancer treatment and follow-up care.
The ACA ensures cancer patients participating in clinical trials will be covered. It also removes annual and lifetime maximums that insurance plans would pay for cancer patients. It prohibit insurance companies from dropping patients with life-threatening diseases like mesothelioma, and it allows patients with pre-existing conditions, like cancer, to obtain coverage with a health insurance plan.
VA Benefits and Insurance
Military veterans often are eligible for benefits under the U.S. Veterans Health Administration, a national network that includes Dr. Robert Cameron in Los Angeles and Dr. Abraham Lebenthal in Boston, two prominent mesothelioma specialists.
Veterans account for an inordinate amount of mesothelioma patients. They are eligible to see mesothelioma specialists wherever they choose, provided their local doctor agrees. And their out-of-pocket expenses should be minimal in the VA system. Asbestos.com has a veteran’s counselor who can help you work through the system and arrange travel grants to make it happen.
Insurance and Diagnostics
Not only is mesothelioma difficult to treat, but it also is difficult to diagnose, which means multiple tests to confirm a diagnosis. The early symptoms often mirror those of less serious illnesses, which lead to a battery of tests, many which must be approved by insurance plans.
The diagnosis might start with X-rays, followed by an MRI and later CT scans and PET scans — all designed to eliminate other possible causes. Fluid and tissue samples are later obtained through biopsies to confirm any suspicions of mesothelioma. Those can be done multiple ways, depending on the doctor performing them.
Biopsies can be done through a thoracoscopy, which involves a camera equipped tube that is inserted through the chest wall or a fine needle aspiration. There also are excisional biopsies, which include tumor cell samples.
A mesothelioma specialist, who knows exactly what to look for, often can streamline the process, making the insurance issue easier.
Choosing the right medical specialists
Mesothelioma or cancer patients know they need to consult with a surgeon or oncologist once diagnosed. However, not all doctors are qualified to handle mesothelioma. Finding a medical team that specializes in mesothelioma is the most important decision a patient can make. These specialists know about the newest, most promising treatment options for the best prognosis possible.
What Mesothelioma Specialists Offer :
Skill
Specialists have the ability to diagnose mesothelioma and develop unique treatment plans for every patient they see, based on each diagnosis.
Experience
Mesothelioma is a rare disease that few doctors encounter in their careers. Specialists, however, see multiple patients every year, making them familiar with mesothelioma and its treatment.
Why Experience Matters
The ideal specialist has extensive experience in a broad range of mesothelioma diagnoses and treatments. There are many factors that contribute to a mesothelioma specialist’s ability to treat the disease effectively.
Connections
Good doctors are connected with top cancer centers, clinical trials and the latest treatments. This makes it easier to receive an integrated treatment experience from people who understand the disease.
Years in Practice
When it comes to caring for patients with mesothelioma, experience counts. Mesothelioma is a rare disease with unique challenges. Seasoned specialists with years of experience know what to expect and how to handle it.
Multi-Disciplinary Approach
It’s an advantage for patients to be treated by a team of people that are all working together towards a common goal. Receiving treatment at a cancer center that can perform all disciplines of treatment in one location protects patients from communication problems between medical professionals. It also provides patients with an expert panel of doctors who can work as a team to create a plan that incorporates multiple disciplines of care to improve life expectancy as much as possible.
Specific Mesothelioma Types
Mesothelioma occurs in the lungs, abdomen, or heart. Having a doctor who specializes in the location of a patient’s diagnosis is very important. Some specialists focus on pleural mesothelioma, while others specialize in peritoneal mesothelioma. Specialists are not one-size-fits-all and must be selected based on the patient’s diagnosis.
Treatment for Different Stages
Some doctors develop personalized treatment plans for every cancer stage. Other specialists focus on providing pain relief and life extension, while yet others are experts in curative options. What a patient needs is a team of physicians that can do all of the above comfortably and have experience with their unique diagnosis.
Surgical Experience
There are a wide variety of surgical options for mesothelioma patients, ranging from the chest to the abdominal region to the heart. Not every specialist has experience with all areas, so finding a specialist with experience in your affected area is vital.
Treatment Adjustment
Because of the nature of mesothelioma, treatment plans often require some adjustment. Specialists are able to monitor improvement and effectiveness, and then prescribe new treatment if necessary. Patients should seek specialists who are willing to monitor and adjust their treatment plans.
Benefits of considering the right Specialist
Choosing a specialist who can cater to a patient’s specific type and location of mesothelioma is important. A patient should be comfortable communicating with their doctor.
Bedside Manner
Great healthcare is not all science; it’s also an art. For some patients, having a specialist who can provide emotional support and trust is extremely important. Many patients prefer doctors who help them understand what they are going through; doctors who have the hands of a surgeon and the heart of a friend.
Clinical Trials
Many specialists are well connected with the clinical trial community and can assist patients in finding the most advanced trials. Admittance into clinical trials is a great way to receive cutting edge treatments. Mesothelioma specialists can be a gateway to these important options as they are often the overseeing physicians in these trials.
Insurance Acceptance
Mesothelioma treatment can be very expensive, and for that reason alone, finding a specialist that accepts a patient’s insurance is critical. Financial aid options that go beyond the cost of treatment may also be available. Patients should keep their ear to the ground with regard to new healthcare options since the laws are changing with new installments of the Affordable Care Act.
Location and Ease of Travel
Many people travel across the country to see top specialists. There are very few treatment programs dedicated to mesothelioma, so travel is something to consider when looking for cutting edge opportunities. Certain programs even provide lodging for families while the patient undergoes treatment.
Types of Specialists
Pre-Diagnosis Specialists
- General physician – Often a family doctor who performs basic tests and imaging (such as X-rays), then refers a patient to a specialist based on the results
- Radiologist – Focuses on imaging technologies (X-ray, CAT scan, PET scan, and CT scan) used in the diagnosis of disease
- Pathologist – Reviews fluid or tissue biopsy samples under a microscope to determine cell type
- Pulmonologist – Specializes in lung disease and evaluates lung function
- Gastroenterologist – Specializes in diseases of the digestive system and issues occurring in the abdominal region
- Surgeon – Performs a surgical biopsy which is sent to a pathologist for review. A thoracic surgeon performs pleural biopsies and a general surgeon performs peritoneal biopsies
- Cardiologist – Specializes in congenital heart defects and other heart disorders
Post Diagnosis Specialists
- Medical oncologist – Prescribes treatment after cancer is diagnosed. A general oncologist is the first team member a patient sees
- Radiation oncologist – Performs non-surgical radiation therapy
- Respiratory therapist – Supports respiratory functions and recovery following surgery
- Thoracic surgeon – Performs invasive surgery on the chest and lung region to remove cancer cells
- Surgical oncologist – Performs operations on areas of the body affected by cancer (including the abdomen) to surgically remove tumors and cancer cells
- Palliative care specialist – Provides treatments related to pain relief, patient comfort, and general well-being
- Psychologist – Provides treatment for the patient’s emotional needs, including counseling for patients and their families
REFERENCE AND SOURCES
- blf.org.uk
- mayoclinic.org
- gov.uk
- asbestos.com
- asbestosnetwork.com
- mesothelioma-mesothelioma.com
- ncbi.nlm.nih.gov
- healthline.com
- medscape.com
- impactlaw.com
- mesothelioma.com
- mesotheliomaguide.com
- news-medical.net
- pleuralmesothelioma.com












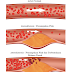



0 comments:
Posting Komentar